Protein Stability Analysis: Ensuring Functionality and Longevity in Biopharmaceuticals and Beyond
What Is Protein Stability Analysis and Why Does It Matter?
Protein stability analysis is a critical process that assesses the structural integrity and functional longevity of a protein under various environmental conditions. Stability is essential for understanding how a protein will perform, especially in pharmaceutical and industrial applications where proteins are often exposed to different pH levels, temperatures, or mechanical stresses. Stable proteins maintain their structure and biological function over time, which is vital in drug development and manufacturing processes, where any degradation or unfolding could compromise efficacy and safety. Protein stability is also essential in research and therapeutic applications since an unstable protein may lose activity, aggregate, or trigger immune responses. Stability analysis thus helps ensure that proteins retain their desired properties under practical use conditions, facilitating the creation of robust and effective protein-based products.How Is Protein Stability Measured?
Various methods are used to assess protein stability, with each technique offering insights into different aspects of structural integrity. Differential Scanning Calorimetry (DSC) measures the heat absorbed or released as a protein unfolds, providing a direct assessment of thermal stability. This method is valuable for determining the melting temperature (Tm), a key indicator of a protein's thermal resilience. Circular Dichroism (CD) Spectroscopy is another widely used technique that assesses secondary structure changes by measuring how proteins absorb circularly polarized light. CD spectroscopy is sensitive to minor conformational changes and can detect unfolding or aggregation events. Dynamic Light Scattering (DLS) is used to evaluate protein aggregation by measuring particle size in solution, offering insights into a protein's propensity to aggregate over time. Fluorescence-based techniques, such as intrinsic fluorescence or Förster resonance energy transfer (FRET), provide high sensitivity to detect even minor unfolding events by monitoring the environment of aromatic residues like tryptophan. These techniques, often used in combination, provide a comprehensive view of a protein's stability, allowing researchers to identify vulnerabilities and optimize formulation conditions accordingly.How Does Protein Stability Analysis Benefit Drug Development and Biotechnology?
In biopharmaceuticals, protein stability is paramount, as therapeutic proteins and antibodies need to remain stable and active throughout manufacturing, storage, and administration. Stability analysis allows developers to predict and mitigate potential degradation pathways, ensuring that biologic drugs retain their efficacy and do not form harmful aggregates. For example, antibody-based drugs undergo rigorous stability testing to assess their behavior under various pH levels and temperatures encountered during processing and storage. Stability analysis also aids in optimizing formulation conditions, such as choosing suitable excipients, stabilizers, and buffers that enhance protein stability. Beyond pharmaceuticals, protein stability is crucial in industrial enzymes used in detergents, food processing, and biofuels, where they must withstand extreme conditions while maintaining activity. By analyzing stability, researchers can engineer proteins to increase their robustness, ensuring that they perform effectively in real-world applications and have a longer shelf life. This meticulous approach to stability ensures that protein-based products meet quality and safety standards across a wide range of industries.What Are the Key Drivers in the Protein Stability Analysis Market?
The growth in the protein stability analysis market is driven by several factors, including advancements in analytical technology, increasing demand for biologics, and an emphasis on quality control. First, technological innovations in stability analysis, such as high-throughput screening, label-free methods, and automated systems, have made it faster and more efficient to analyze protein stability. These advancements enable detailed stability profiling during early-stage drug development, allowing for better candidate selection and reducing the risk of late-stage failures. Additionally, the growing demand for biologics and biosimilars, especially in treating complex diseases like cancer and autoimmune disorders, has intensified the need for rigorous stability testing to ensure product efficacy and safety. Consumer expectations for high-quality protein-based products in sectors like food, cosmetics, and healthcare further drive this demand, particularly as regulatory agencies like the FDA and EMA enforce strict guidelines for biologics' stability. Lastly, the expansion of bioprocessing and industrial biotechnology requires robust proteins with enhanced stability for consistent performance, stimulating continued investment in protein stability analysis tools and technologies. These factors collectively underscore a market where precision in protein stability is essential for product success, safety, and market competitiveness.Report Scope
The report analyzes the Protein Stability Analysis market, presented in terms of market value (US$ Thousand). The analysis covers the key segments and geographic regions outlined below.- Segments: Product (Reagents & Assay Kits, Instruments, Consumable & Accessories, Software); Technique (Chromatography, Spectroscopy, Surface Plasma Resonance Imaging (SPRI), Differential Scanning Calorimetry (DSC), Differential Scanning Fluorimetry (DSF), Other Techniques); End-Use (Pharma & Biotech Companies, Contract Research Organizations, Academic & Research Institutes).
- Geographic Regions/Countries:World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Reagents & Assay Kits segment, which is expected to reach US$2.1 Billion by 2030 with a CAGR of a 9.5%. The Instruments segment is also set to grow at 7% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $623.8 Million in 2024, and China, forecasted to grow at an impressive 13% CAGR to reach $1.1 Billion by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Protein Stability Analysis Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Protein Stability Analysis Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Protein Stability Analysis Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as Agilent Technologies, Inc., Enzo Biochem, Inc., General Electric, Horiba, Ltd, Perkinelmer, Inc. and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 32 companies featured in this Protein Stability Analysis market report include:
- Agilent Technologies, Inc.
- Enzo Biochem, Inc.
- General Electric
- Horiba, Ltd
- Perkinelmer, Inc.
- Setaram Instrumentation
- Shimadzu Corporation
- Spectris PLC
- Thermo Fisher Scientific
- Unchained Labs
- Waters Corporation
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Agilent Technologies, Inc.
- Enzo Biochem, Inc.
- General Electric
- Horiba, Ltd
- Perkinelmer, Inc.
- Setaram Instrumentation
- Shimadzu Corporation
- Spectris PLC
- Thermo Fisher Scientific
- Unchained Labs
- Waters Corporation
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 377 |
| Published | January 2026 |
| Forecast Period | 2024 - 2030 |
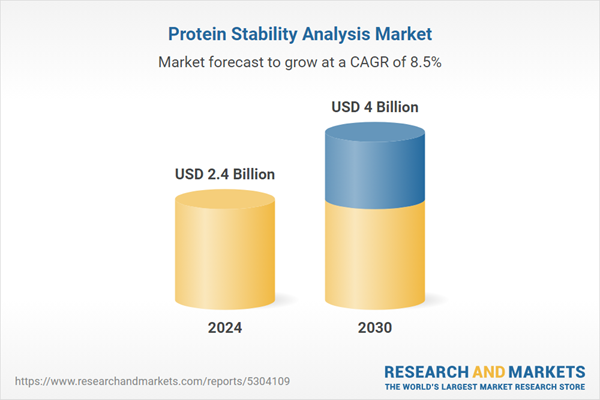
| Estimated Market Value ( USD | $ 2.4 Billion |
| Forecasted Market Value ( USD | $ 4 Billion |
| Compound Annual Growth Rate | 8.5% |
| Regions Covered | Global |