Global Alpha 1 Antitrypsin Deficiency Treatment Market - Key Trends & Drivers Summarized
What Is Alpha 1 Antitrypsin Deficiency and Why Is It Important?
Alpha 1 Antitrypsin Deficiency (AATD) is a genetic disorder that affects the lungs and liver, caused by insufficient levels of the protein alpha 1 antitrypsin (AAT), which protects tissues from enzyme damage. The deficiency can lead to chronic obstructive pulmonary disease (COPD), emphysema, and liver diseases like cirrhosis. AATD is underdiagnosed, and many patients suffer from symptoms for years before receiving appropriate treatment. Managing the condition often requires augmentation therapy, where patients are given purified AAT to reduce lung damage and prevent further deterioration.What Recent Advancements Are Improving Alpha 1 Antitrypsin Deficiency Treatments?
Recent advancements in the treatment of Alpha 1 Antitrypsin Deficiency include the development of new augmentation therapies and research into gene therapies aimed at correcting the underlying genetic cause. Augmentation therapy, which involves intravenous administration of purified AAT derived from human plasma, remains the primary treatment option for AATD. However, researchers are now focusing on more advanced therapeutic approaches such as RNA-based therapies, which aim to correct the faulty gene responsible for AATD. These developments are creating more effective and long-term solutions for patients, potentially reducing the need for lifelong augmentation therapy.How Is Awareness and Diagnosis Impacting AATD Treatment Trends?
One of the key challenges in treating Alpha 1 Antitrypsin Deficiency is the high rate of underdiagnosis. Increased awareness campaigns by healthcare organizations and patient advocacy groups are gradually improving diagnosis rates, leading to earlier intervention and better outcomes for patients. Genetic testing has also become more widely available, allowing individuals with a family history of AATD to be screened and diagnosed earlier. As awareness of AATD grows, more patients are gaining access to life-saving treatments, particularly in developed healthcare markets.What Factors Are Driving the Growth of the Alpha 1 Antitrypsin Deficiency Treatment Market?
The growth in the Alpha 1 Antitrypsin Deficiency treatment market is driven by several factors, including advancements in gene and augmentation therapies, improved diagnosis rates, and increasing awareness of the condition. Innovations in augmentation therapy and research into genetic correction methods are making treatments more effective and accessible. The growing availability of genetic testing is also helping in early detection and treatment, significantly improving patient outcomes. Moreover, patient advocacy and education campaigns are raising awareness about the disorder, prompting earlier intervention and driving demand for AATD therapies.Report Scope
The report analyzes the Alpha 1 Antitrypsin Deficiency Treatment market, presented in terms of market value (US$ Thousand). The analysis covers the key segments and geographic regions outlined below.- Segments: End-Use (Specialty Clinics, Hospitals, Pharmacies); Route of Administration (Parenteral, Inhalation, Oral); Treatment (Augmentation Therapy, Bronchodilator, Corticosteroids, Oxygen Therapy, Other Treatments).
- Geographic Regions/Countries:World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; and Rest of Europe); Asia-Pacific; Rest of World.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Alpha 1 Antitrypsin Deficiency Treatment Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Alpha 1 Antitrypsin Deficiency Treatment Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Alpha 1 Antitrypsin Deficiency Treatment Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as Abeona Therapeutics Inc., Applied Genetic Technologies Corporation, Arrowhead Pharmaceuticals Inc., AstraZeneca PLC, Baxter International, Inc. and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 46 companies featured in this Alpha 1 Antitrypsin Deficiency Treatment market report include:
- Abeona Therapeutics Inc.
- Applied Genetic Technologies Corporation
- Arrowhead Pharmaceuticals Inc.
- AstraZeneca PLC
- Baxter International, Inc.
- Biogen, Inc.
- Boehringer Ingelheim International GmbH
- Chiesi Farmaceutici SpA
- CSL Behring
- Curaxys
- GlaxoSmithKline PLC
- Grifols International SA
- Kamada Ltd.
- Pfizer, Inc.
- ProBioGen AG
- ProMetic Life Sciences, Inc.
- Shire PLC
- Teva Pharmaceutical Industries Ltd.
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Abeona Therapeutics Inc.
- Applied Genetic Technologies Corporation
- Arrowhead Pharmaceuticals Inc.
- AstraZeneca PLC
- Baxter International, Inc.
- Biogen, Inc.
- Boehringer Ingelheim International GmbH
- Chiesi Farmaceutici SpA
- CSL Behring
- Curaxys
- GlaxoSmithKline PLC
- Grifols International SA
- Kamada Ltd.
- Pfizer, Inc.
- ProBioGen AG
- ProMetic Life Sciences, Inc.
- Shire PLC
- Teva Pharmaceutical Industries Ltd.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 196 |
| Published | February 2026 |
| Forecast Period | 2024 - 2030 |
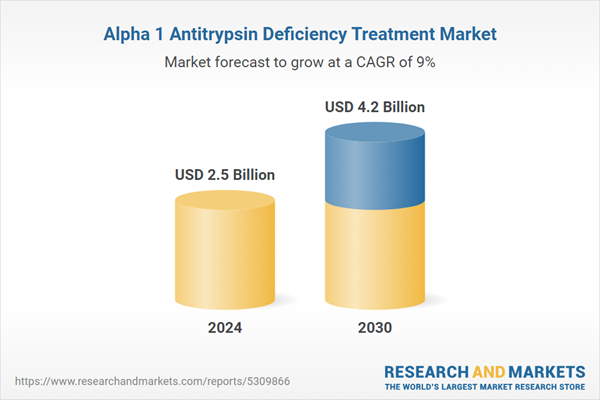
| Estimated Market Value ( USD | $ 2.5 Billion |
| Forecasted Market Value ( USD | $ 4.2 Billion |
| Compound Annual Growth Rate | 9.0% |
| Regions Covered | Global |