Global Rare Hematology Market - Key Trends & Drivers Summarized
What Is The Rare Hematology Market and Why Is It Gaining Momentum in Healthcare?
The Rare Hematology market focuses on the diagnosis, treatment, and management of rare blood disorders, which include conditions such as hemophilia, sickle cell disease, thalassemia, paroxysmal nocturnal hemoglobinuria (PNH), and various types of anemia. These diseases are classified as rare because they affect a small percentage of the global population, typically less than 1 in 2,000 individuals. Although the patient population for each rare hematologic condition is relatively small, the cumulative impact of these diseases on healthcare systems is significant due to their chronic nature, complex management requirements, and the high cost of treatments. Traditionally, patients with rare hematologic conditions have faced limited treatment options and low levels of research investment. However, recent advancements in genomics, biotechnology, and personalized medicine are changing this scenario, leading to a surge in research and development activities aimed at discovering novel therapies and improving patient outcomes. As a result, the Rare Hematology market is gaining momentum, driven by increasing awareness, supportive policies, and the introduction of cutting-edge treatments that are reshaping the management of these challenging conditions.The rise of patient advocacy groups and increased collaboration between healthcare providers, pharmaceutical companies, and regulatory bodies have also contributed to the growth of the Rare Hematology market. Organizations such as the National Organization for Rare Disorders (NORD) and the European Hematology Association (EHA) have been instrumental in advocating for more research funding, better access to treatments, and enhanced patient care. These efforts have spurred pharmaceutical companies to invest in the development of orphan drugs - medications designed to treat rare conditions - despite the smaller market size. Governments around the world have introduced various incentives, including market exclusivity, tax credits, and fast-track approval processes, to encourage the development of these specialized treatments. As a result, the Rare Hematology market has seen a steady influx of new therapeutic options, ranging from gene therapies and monoclonal antibodies to advanced biologics. With the advent of these innovative solutions, healthcare providers are better equipped to manage rare hematologic disorders, improving the quality of life for patients who previously had limited or no treatment options.
How Are Technological Advancements Transforming The Treatment Landscape Of Rare Hematologic Disorders?
Technological advancements have played a transformative role in the development of therapies for rare hematologic disorders, revolutionizing the way these diseases are diagnosed and treated. One of the most groundbreaking innovations in this field is the advent of gene therapy. Gene therapies target the underlying genetic mutations responsible for many rare blood disorders, offering the potential for long-term or even curative treatments. For example, in the treatment of hemophilia, gene therapies aim to introduce a functional copy of the defective gene, enabling patients to produce the necessary clotting factors naturally, thereby reducing or eliminating the need for regular factor replacement therapy. Similarly, gene-editing technologies such as CRISPR-Cas9 are being explored as potential treatments for sickle cell disease and beta-thalassemia, with early clinical trials showing promising results. These technologies have the potential to transform the treatment paradigm for rare hematologic disorders, offering hope for patients who have historically relied on symptomatic treatments.In addition to gene therapy, advances in biologics and targeted therapies are expanding the range of treatment options available for rare hematologic conditions. Monoclonal antibodies, for instance, have been developed to target specific pathways or proteins involved in disease progression, offering a more precise approach to treatment with fewer side effects. For conditions like PNH, targeted therapies such as complement inhibitors have been introduced, which effectively reduce hemolysis and the risk of thrombosis, significantly improving patient outcomes. Furthermore, the integration of personalized medicine into rare hematology is enabling healthcare providers to tailor treatments based on individual genetic profiles, ensuring a higher degree of efficacy and safety. Diagnostic technologies are also evolving, with next-generation sequencing (NGS) and high-throughput screening methods making it possible to identify rare genetic mutations with greater accuracy and speed. These technological advancements are not only enhancing the precision of diagnoses but also facilitating earlier detection and intervention, which are crucial for managing rare hematologic disorders more effectively.
What Factors Are Driving The Adoption Of New Treatments In The Rare Hematology Market?
Several factors are contributing to the increased adoption of new treatments in the Rare Hematology market, including the growing prevalence of rare blood disorders, advancements in medical research, and favorable regulatory frameworks. The rising incidence of rare hematologic conditions is partly due to improved diagnostic capabilities, which have led to higher detection rates and more accurate classification of these diseases. With more patients being accurately diagnosed, there is a greater demand for innovative therapies that go beyond conventional treatments. At the same time, advancements in medical research, particularly in the fields of genomics and biotechnology, are enabling the development of more effective and targeted therapies. The success of these therapies in clinical trials has spurred pharmaceutical companies to invest heavily in rare hematology, leading to an increase in the availability of new and improved treatment options for patients.Another key driver of treatment adoption is the supportive regulatory environment. Recognizing the unique challenges associated with developing therapies for rare diseases, regulatory bodies such as the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) have established special designations like Orphan Drug Status and Breakthrough Therapy Designation. These programs provide incentives such as extended market exclusivity, expedited review processes, and reduced regulatory fees, making it more attractive for companies to pursue drug development in the rare hematology space. Additionally, the growing influence of patient advocacy groups has led to increased awareness and better access to information about available treatments, empowering patients and their families to seek out specialized care and novel therapies. As a result, there is a rising acceptance of these new treatments, especially among patient populations that previously had limited or no therapeutic options. The combination of these factors is driving higher adoption rates and expanding the reach of new therapies within the Rare Hematology market.
What Is Driving The Growth Of The Global Rare Hematology Market?
The growth in the global Rare Hematology market is driven by several factors, including the increased focus on rare disease research, advancements in therapeutic technologies, and evolving healthcare policies. One of the primary growth drivers is the rising investment in research and development by pharmaceutical and biotechnology companies. Encouraged by favorable regulatory incentives and growing patient demand, companies are dedicating substantial resources to the discovery and development of innovative therapies for rare blood disorders. This has led to a steady pipeline of new treatments, ranging from gene therapies and enzyme replacements to next-generation biologics and small molecule drugs. The market is also benefiting from technological innovations that are enabling more effective disease modeling, drug screening, and clinical trials, thereby accelerating the development process and bringing new therapies to market more quickly.Evolving healthcare policies are another crucial driver of market growth. Many countries have introduced national rare disease plans or policies aimed at improving the diagnosis, treatment, and care of patients with rare diseases, including hematologic conditions. These policies often include initiatives to increase research funding, support the development of specialized care centers, and improve patient access to advanced therapies. Furthermore, collaborations between academia, industry, and patient organizations are fostering a more collaborative research environment, accelerating the pace of innovation in the field. Another growth driver is the increasing prevalence of rare hematologic conditions, which is creating a larger patient pool in need of specialized treatments. Improved awareness and better diagnostic capabilities are contributing to higher diagnosis rates, which in turn are driving demand for targeted therapies and comprehensive care solutions. As these factors converge, the global Rare Hematology market is poised for continued growth, supported by a strong innovation pipeline, enhanced regulatory frameworks, and an ever-increasing focus on improving patient outcomes and quality of life.
Report Scope
The report analyzes the Rare Hematology market, presented in terms of market value (US$ Thousand). The analysis covers the key segments and geographic regions outlined below.- Segments: Product Type (Plasma Derived Factors, Recombinant Factors); Age Group (Adult (18+), Pediatric (0-17)).
- Geographic Regions/Countries:World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; and Rest of Europe); Asia-Pacific; Rest of World.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Plasma Derived Factors segment, which is expected to reach US$18.2 Billion by 2030 with a CAGR of a 5%. The Recombinant Factors segment is also set to grow at 4.6% CAGR over the analysis period.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Rare Hematology Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Rare Hematology Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Rare Hematology Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as Alexion Pharmaceuticals, Inc., Bayer Healthcare AG, CSL Behring, Novo Nordisk A/S, PRA Health Sciences and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 42 companies featured in this Rare Hematology market report include:
- Alexion Pharmaceuticals, Inc.
- Bayer Healthcare AG
- CSL Behring
- Novo Nordisk A/S
- PRA Health Sciences
- Shire PLC
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Alexion Pharmaceuticals, Inc.
- Bayer Healthcare AG
- CSL Behring
- Novo Nordisk A/S
- PRA Health Sciences
- Shire PLC
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 194 |
| Published | February 2026 |
| Forecast Period | 2024 - 2030 |
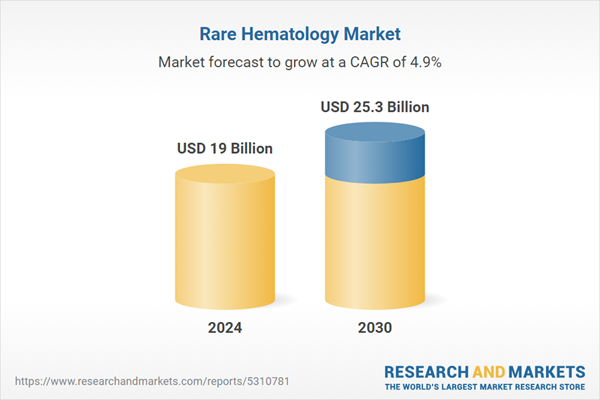
| Estimated Market Value ( USD | $ 19 Billion |
| Forecasted Market Value ( USD | $ 25.3 Billion |
| Compound Annual Growth Rate | 4.9% |
| Regions Covered | Global |