Rising Number of Clinical Trials
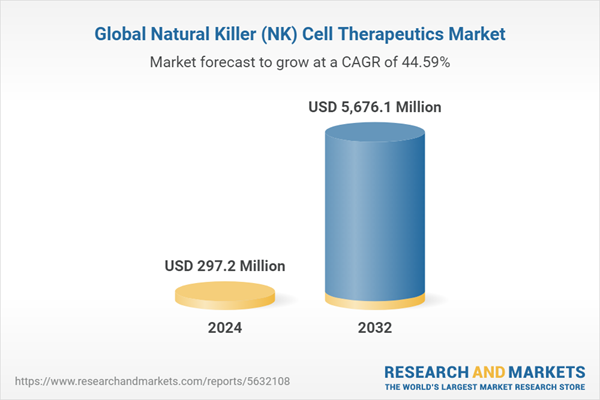
The global NK cell therapeutics market is expected to reach $5,676.1 million in 2032 from $297.2 million in 2024 at a CAGR of 44.59% during the forecast period 2024-2032. The growth in the global NK cell therapeutics market is expected to be driven by the rising in the number of clinical trials and the increasing incidence and prevalence of cancer cases.
Market Lifecycle Stage
The global NK cell therapeutics market is at a very nascent stage. Currently, there is no approved NK cell therapy in the market. Many biopharmaceutical companies are trying to research and develop new immunotherapies, especially NK cell therapies which would be cost-effective and hence, could act as a next-generation medication for cancer and other acute infectious indications. For instance, major players such as Affimed N.V., GC Cell (GC Biopharma corp.), Glycostem Therapeutics B.V., and Takeda Pharmaceutical Company Limited are investing heavily in clinical trials for their respective therapeutic candidates for various indications such as acute myeloid leukemia (AML), B-cell non-Hodgkin’s lymphoma (NHL) - relapsed or refractory, hepatocellular carcinoma (HCC), and peripheral T-cell lymphoma. Increasing funding and investments in research and development is one of the major opportunities in the global NK cell therapeutics market.
Impact
With an increased worldwide focus on researching NK cell therapies, the major market players are developing novel therapeutics, such as NK cells therapy (unmodified), CAR-NK cells therapy (modified), and NK cell engager. For instance, currently, there are 79 ongoing clinical trials for NK cell therapeutics for different types of indications such as acute myeloid leukemia, hematologic malignancies, hepatocellular carcinoma, and peripheral T-cell lymphoma, which is significantly impacting the growth of NK cell therapeutics market.
Immunotherapies are more prominent in countries such as the U.S., the U.K., and South Korea. Moreover, the presence of major market players such as Affimed N.V., Fate Therapeutics, Inc., Gamida Cell Ltd., GC Cell (GC Biopharma corp.), Nkarta, Inc., Sanofi S.A., and Takeda Pharmaceutical Company Limited in eight major countries, including the U.S., EU5 (U.K., Germany, France, Spain, Italy), Japan, and South Korea have a positive impact on the market growth.
Impact of COVID-19
Due to the pandemic, governments across the globe enforced lockdowns which significantly hampered the research and clinical development of NK cell therapeutics. This situation was majorly witnessed till Q2 2021, which created a negative impact on the global NK cell therapeutics market, resulting in a decline in preclinical and clinical trials of the product candidates. The major reason has been the difficulty in patient recruitment for the ongoing clinical trials, which will have an impact on the launch timelines of the drugs.
However, it is worth mentioning here that with the ease of restrictions after Q2 2021, the market for NK cell therapy has gained significant momentum and is anticipated to grow during the forecast period 2024-2032.
The COVID-19 pandemic has exerted tremendous strain on the research and clinical development of NK cell therapeutics. Companies operating in the field of NK cell therapies have changed their focus on the research and development of drugs used for COVID-19 treatment to help governments and communities establish a new normal across the globe.
This has resulted in the delayed launch of emerging therapies due to a shift in priorities on account of the COVID-19 pandemic. In addition, pharmaceutical companies are diverting their financial resources to develop and repurpose existing drug molecules for the prevention and treatment of COVID-19. This strategy has been adopted by most pharmaceutical companies to generate high revenue from the sale of COVID-19 drugs. These factors exert a combinatorial effect on the development and launch of emerging NK therapies.
For instance, Affimed N.V. faced challenges in the patients’ enrolment due to the COVID-19 pandemic for the investigation of AFM13 therapy in patients suffering from transformed mycosis fungoides (TMF). Hence, the company decided to halt the trial of AFM13 for patients with TMF. Similarly, Celularity Inc. also faced difficulty in the enrolment of the patients for its phase 1 clinical trial of CYNK-001 in early 2020 and again in mid-2021, which was for the indication of acute myeloid leukemia. Furthermore, Nkarta, Inc.’s few clinical trial sites have restricted enrollment for the clinical trials temporarily to prioritize hospital beds for patients suffering from COVID-19. Also, enrolment delays were experienced due to the supply chain and operational disruptions experienced owing to COVID-19. This led to some delays in gathering up the clinical sites for the clinical trials of NKX101 and NKX019.
Market Segmentation
by Therapy Type
- NK Cell Therapy (Unmodified)
- CAR-NK Cell Therapy (Modified)
- NK Cell Engagers
In the global NK cell therapeutics market, the NK cell therapy (unmodified) segment is anticipated to dominate the global NK cell therapeutics market during the forecast period and is expected to hold a share of 57.82%. The segment includes the pipeline NK cell therapeutics derived from allogenic or off-the-shelf cancer-killing immunotherapy, which is readily available from cord blood supplies.
by Pipeline Products
- AFM13
- MG4101
- GTA002 (oNKordA)
- TAK-007
In 2032, the global NK cell therapeutics market is estimated to be dominated by MG4101 and is expected to hold a 36.35% share of total NK cell therapeutics market, followed by TAK-007 with the market share of 24.20% of the NK cell therapeutics market in 2032.
by Indication
- Acute Myeloid Leukemia
- Relapsed or Refractory (r/r) B-cell Non-Hodgkin’s Lymphoma (NHL)
- Hepatocellular Carcinoma
- Peripheral T-Cell Lymphoma
The B-cell non-Hodgkin’s lymphoma - relapsed or refractory segment is expected to dominate the global NK cell therapeutics market in 2032 due to the rise in B-cell lymphoma patients across the countries and high unmet need in the disease area.
by Country
- U.S.
- U.K
- France
- Spain
- Italy
- Germany
- Japan
- South Korea
The U.S. NK cell therapeutics market will be valued at $232.4 million in 2024 and is expected to be the leading market contributor. The growth can be attributed to the increased research and development activities in the country.
Recent Developments in the Global NK Cell Therapeutics Market
- In April 2022, Affimed N.V. announced data on the dose-escalation study of its innate cell engager AFM24 in patients with EGFR-positive solid tumors. As per the data, AFM24 showed a well-managed safety profile.
- In April 2022, Nkarta, Inc. announced positive preliminary data of phase 1 of its two cell therapy candidates, NKX101 and NKX019, of chimeric antigen receptor (CAR) natural killer (NK) cell therapy. The results of the study showed the safety advantages of NK cells with an off-the-shelf modality which was designed to increase the benefits of cell therapy.
- In January 2022, Affimed N.V. announced the completion of the participants for its phase 2 study for its lead product AFM13 as a monotherapy for patients with relapsed or refractory CD30-positive peripheral T-cell lymphoma (PTCL).
- In December 2021, Innate Pharma SA disclosed that the first patient received treatment in a phase 1/2 clinical trial of IPH6101/SAR443579, a lead molecular target for NK cells that targets NKp46/CD16, in patients with chronic myelogenous leukemia (CRML), B-cell acute lymphoblastic leukemia (B-ALL), or high-risk myelodysplastic syndrome (HR-MDS). The trial is being conducted in collaboration with Sanofi S.A.
Demand - Drivers and Limitations
Following are the demand drivers for the global NK cell therapeutics market:
- Rising Number of Clinical Trials
- Increasing Incidence and Prevalence of Cancer
The market is expected to face some limitations too due to the following challenges:
- Lack of Specificity and Poor In Vivo Survival of the NK Cells
- High Cost of Immunotherapy Treatment Associated with Cancer
How Can This Report Add Value to an Organization?
Product/Innovation Strategy: The product segment helps the reader to understand the pipeline products in the market that are in different stages of clinical trials. Additionally, it helps to understand the multiple indications for which the trials are going on along with their route of administration. Moreover, the study provides the reader with a detailed understanding of four different indications such as acute myeloid leukemia (AML), B-cell non-Hodgkin’s lymphoma (NHL) - relapsed or refractory, hepatocellular carcinoma (HCC), and peripheral T-cell lymphoma.
Growth/Marketing Strategy: The global NK cell therapeutics market has seen major development by key players operating in the market, such as regulatory and legal activities, product approvals, partnerships and alliances, and merger and acquisition activities. The favored strategy for the companies has been clinical developments along with regulatory and legal activities. The companies are focusing on enhancing the clinical trials of the NK cell therapies so as to receive the regulatory authorizations as much as possible to strengthen their position in the market. For instance, in January 2020, Fate Therapeutics, Inc. announced that the U.S. Food and Drug Administration (FDA) had cleared the investigational new drug (IND) application for FT536, a chimeric antigen receptor (CAR) NK cell candidate. The company further plans to focus on the clinical investigation of FT536 as a monotherapy and combination for the treatment of multiple solid tumor indications.
Competitive Strategy: Key players in the global NK cell therapeutics market analyzed and profiled in the study involve immunotherapies, especially NK cell therapy. Moreover, a detailed competitive benchmarking of players operating in the global NK cell therapeutics market has been done to help the reader understand how players stack against each other, presenting a clear market landscape. Additionally, comprehensive competitive strategies such as partnerships, agreements, and collaborations will aid the reader in understanding the untapped revenue pockets in the market.
Key Market Players and Competition Synopsis
The companies that are profiled have been selected based on inputs gathered from primary experts and analyzing company coverage, pipeline product portfolio, and market penetration.
Some prominent names established in this market are:
- Affimed N.V.
- Century Therapeutics, Inc.
- Celularity Inc.
- Cytovac A/S
- Dragonfly Therapeutics, Inc.
- Fate Therapeutics, Inc.
- Gamida Cell Ltd.
- GC Cell (GC Biopharma corp.)
- Glycostem Therapeutics B.V.
- ImmunityBio, Inc.
- Innate Pharma SA
- Nkarta, Inc.
- Sanofi S.A.
- Takeda Pharmaceutical Company Limited
- VaxCell Biotherapeutics Co., Ltd.
- Acepodia Inc.
- iCell Gene Therapeutics
- Senti Biosciences
- Shoreline Biosciences
Table of Contents
Companies Mentioned
- Affimed N.V.
- Century Therapeutics, Inc.
- Celularity Inc.
- Cytovac A/S
- Dragonfly Therapeutics, Inc.
- Fate Therapeutics, Inc.
- Gamida Cell Ltd.
- GC Cell (GC Biopharma corp.)
- Glycostem Therapeutics B.V.
- ImmunityBio, Inc.
- Innate Pharma SA
- Nkarta, Inc.
- Sanofi S.A.
- Takeda Pharmaceutical Company Limited
- VaxCell Biotherapeutics Co., Ltd.
- Acepodia Inc.
- iCell Gene Therapeutics
- Senti Biosciences
- Shoreline Biosciences
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 207 |
| Published | June 2022 |
| Forecast Period | 2024 - 2032 |
| Estimated Market Value ( USD | $ 297.2 Million |
| Forecasted Market Value ( USD | $ 5676.1 Million |
| Compound Annual Growth Rate | 44.6% |
| Regions Covered | Global |
| No. of Companies Mentioned | 19 |