Biosimilar medicines are biotherapeutic products that are identical to an approved reference product in terms of quality, safety, and efficacy. Chronic diseases like cancer and autoimmune disorders are treated with biologics. Biologic drug development is an expensive and time-consuming process, but the development of biosimilars saves time & resources by avoiding needless repetition of clinical trials.
Biopharmaceutical brand-name drugs' generic, non-patented chemical & therapeutic equivalents are known as biosimilars or biogenetics. Proteins like enzymes or antibodies, as well as nucleic acids like DNA or RNA, are examples of natural biologic substances. Biopharmaceuticals are synthetic or recombinant versions of these substances. Biogenetics, on the other hand, are not generic biologics because the complicated process of producing biologics prevents there from being a generic form of a biologic.
The prevalence of chronic diseases like diabetes, cancer, and coronary artery diseases as well as the geriatric population is increase all over the world. Additionally, there is a significant increase in number of clinical trials and creative R&D strategies to create advanced drugs. It is anticipated that rising therapeutic applications and increased R&D in the pharmaceutical sector will open up new market opportunities.
COVID-19 Impact Analysis
Lockdowns have been implemented in many nations as a result of the COVID-19 outbreak, it is anticipated that this will have a negative effect on the growth of the biosimilars market. The COVID-19 pandemic made it difficult for the pharmaceutical industry to concentrate on R&D activities, such as the creation of biosimilars. During the pandemic, the entire healthcare sector concentrated on developing life-saving products. Additionally, delays in product approvals, as well as launches brought on by pandemic situations, is expected to limit the growth of the biosimilar market.Market Growth Factors
Blockbuster Biologics' Patents are About to Expire, and New Indications are Being Investigated
The majority of early biologic drugs lost their patent protection in the last few years, however, many of today's top-selling medications are expected to do the same in the upcoming years. This is opening up new possibilities for biosimilar medicines. Biosimilars that are currently on the market are used to treat a variety of illnesses and conditions, such as rheumatoid arthritis, cancer, psoriasis, infectious diseases, anemia, kidney failure, type 1 & type 2 diabetes, postmenopausal osteoporosis, and disorders of the growth hormone.Cost-Effectiveness of Biosimilars
Pharmaceutical preparations known as biologic drugs are either created using living organisms, like bacterial and fungi cultures or mouse animal models, or they contain components that were obtained from them. Medical professionals use these drugs a lot to treat their patients. Biosimilars are administered to patients in the same way as their reference biologics and, in terms of clinical efficacy, produce the same therapeutic effects. However, they differ from the original drug in terms of composition because the latter has a patent owned by a pharmaceutical company that prevents other manufacturers from duplicating it while the patent is in effect.Market Restraining Factors
Challenges Associated With the Manufacturing Process
The creation of biosimilars is a difficult and expensive process that needs a lot of money, technical know-how, scientific standards, clinical trial experience, and quality control procedures. Contrary to the development of generic drugs, manufacturers of biosimilars are obligated to make comparable investments in clinical trials & post-approval safety monitoring programs. The ability to control variability during the manufacturing process, where the final products are comparable to their biological products, is another important challenge in the production of biosimilars.Application Outlook
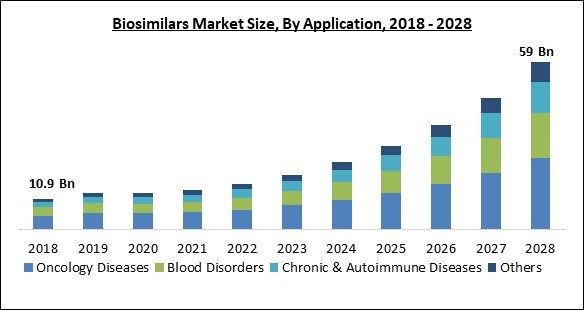
Based on application, the biosimilars market is segmented into blood disorders, oncology diseases, chronic & autoimmune diseases and others. In 2021, the chronic & autoimmune disease segment procured a promising revenue share in the biosimilars market. This is because of an increase in product approvals and a rise in demand for cutting-edge treatment. In addition, the demand for biosimilar products is expected to grow in this segment due to an increase in the number of elderly people, a change in lifestyle, and an unhealthy diet.Type Outlook
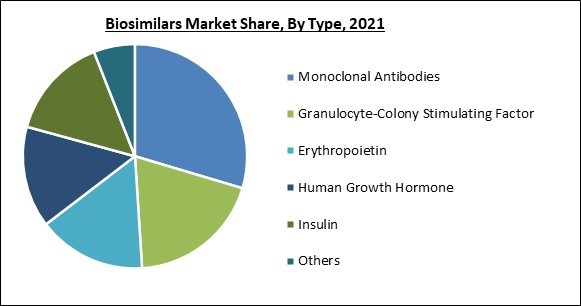
On the basis of type, the biosimilars market is fragmented into human growth hormone, erythropoietin, monoclonal antibodies, insulin, granulocyte-colony stimulating factor, and others. In 2021, the monoclonal antibodies segment held the highest revenue share in the biosimilars market. Man-made monoclonal antibodies function similarly to human antibodies in the immune system. Many diseases, such as some forms of cancer, can be treated with monoclonal antibodies. Due to an increase in R&D activities to develop new biosimilars and a rise in the use of monoclonal antibodies for cancer treatment, the demand for biosimilars in this segment is expected to rise.Regional Outlook
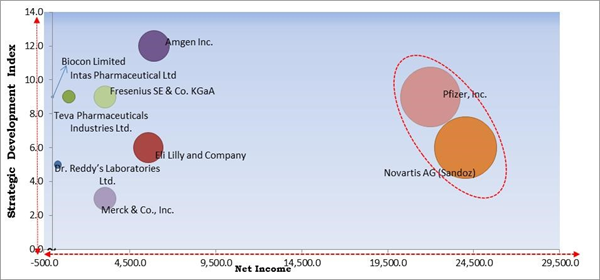
Region wise, the biosimilars market is analyzed across North America, Europe, Asia Pacific, and LAMEA. The North America region led the biosimilars market with the highest revenue share in 2021. This is because of the rising number of cases of the oncology diseases in Canada. Also, the advanced healthcare infrastructure is expected to support the regional market growth. In addition to this, various initiatives taken by the government coupled with the demand for robust medical facilities by the people in the region having high disposable income are supporting the market growth in this region.Cardinal Matrix - Biosimilars Market Competition Analysis
The major strategies followed by the market participants are Partnerships. Based on the Analysis presented in the Cardinal matrix; Pfizer, Inc. and Novartis AG (Sandoz) are the forerunners in the Biosimilars Market. Companies such as Amgen, Inc., Eli Lilly and Company and Fresenius SE & Co. KGaA are some of the key innovators in Biosimilars Market.
The market research report covers the analysis of key stake holders of the market. Key companies profiled in the report include Amgen, Inc., Biocon Limited, Dr. Reddy’s Laboratories Ltd., Eli Lilly and Company, Intas Pharmaceutical Ltd, Novartis AG (Sandoz), Merck & Co., Inc., Pfizer, Inc., Teva Pharmaceuticals Industries Ltd. and Fresenius SE & Co. KGaA
Strategies deployed in Biosimilars Market
; Partnerships, Collaborations and Agreements:
- Aug-2022: Pfizer signed an agreement to acquire Global Blood Therapeutics (GBT), a biopharmaceutical company. This acquisition is expected to improve Pfizer’s more than 30-year heritage in rare hematology & reinforces the company’s commitment to SCD by bringing expertise and a leading portfolio and pipeline with the potential to address the full spectrum of critical requirements in this underserved community.
- Jul-2022: Intas Pharmaceuticals partnered with Prestige Biopharma, a Singapore based biopharmaceutical company. Under this partnership, the commercialization of Prestige Biopharma’s bevacizumab biosimilar, is expected to be supplied in the US, Europe, Canada, MENA, Brazil, Mexico, South Africa, Thailand, Vietnam, Philippines, Malaysia, Singapore, Indonesia, Kyrgyzstan, Tajikistan. This partnership focused on Intas's long-term strategy and is expected to reinforce its commitment on enhancing access to high-quality biosimilar drugs for cancer patients all over the world.
- Apr-2022: Intas Pharmaceuticals signed an agreement with Axantia Holding (Axantia), a leading pharmaceutical company. Under the terms of this agreement, Axantia is expected to register, hold the marketing authorization and commercialize Ranibizumab in certain territories including Saudi Arabia, Jordan, Iraq, Lebanon, and GCC countries. Under this agreement, Axantia is expected to register, hold the marketing authorization & commercialize Ranibizumab in certain territories that Jordan, Saudi Arabia, Iraq, Lebanon, and GCC nations.
- Dec-2021: Dr. Reddy's Laboratories came into a partnership with Prestige BioPharma Ltd, a Singapore-based firm. This partnership aimed at the supply and commercialization of the former's proposed Trastuzumab biosimilar in select countries in Latin America & Southeast Asia. This partnership is expected to help the company combine its established expertise in the area of biosimilars with its commercial strengths and growth ambition in these markets.
- Dec-2021: Eli Lilly and Company collaborated with Regor Therapeutics Group, an international biotech company. Under this collaboration, the companies is expected to discover, develop and commercialize novel therapies for metabolic diseases. The collaboration is expected to provide the opportunity to expand treatment options available to patients suffering from metabolic disorders.
- Dec-2021: Eli Lilly and Company partnered with Innovent Biologics, a world-class biopharmaceutical company. Under this partnership, the companies is expected to the innovative PD-1 inhibitor sintilimab has been successfully included in the updated National Reimbursement Drug List for all approved indications.
- Oct-2021: Eli Lilly and Company came into a partnership with Cipla, an Indian multinational pharmaceutical company. This partnership focused on enhancing the reach of Lilly’s Diabetes products Humalog and Trulicity (Dulaglutide). Under this partnership, Lilly is expected to transfer its rights in India to sell, promote and distribute the aforesaid two Lilly Diabetes products, Humalog & Trulicity, to Cipla, subject to all regulatory approvals.
- Jul-2021: Intas Pharmaceuticals entered into an agreement with Meiji and Dong-A Socio Holdings, a parent company of Dong-A ST. By this agreement, Intas aimed at commercializing DMB-3115, a proposed biosimilar to ustekinumab, a recombinant monoclonal antibody for the treatment of autoimmune and inflammatory diseases like plaque psoriasis, Crohn's disease & ulcerative colitis.
- Jun-2021: Teva Pharmaceutical partnered with Bioeq AG, a Swiss biopharmaceutical company. This partnership expanded Teva’s biosimilar portfolio & again demonstrated the company’s firm commitment of creating greater access to quality medications to help enhance the lives of more patients.
- Feb-2021: Biocon Biologics partnered with Clinton Health Access Initiative, a global health organization. Through this partnership, Biocon focused on expanding access to lifesaving cancer biosimilars in over 30 countries in Asia & Africa. The partnership aimed at delivering advanced cancer therapies to patients who is expected to require them the most & ensuring equitable access to high-quality biosimilars in low- and middle-income nations.
- Oct-2020: Novartis teamed up with Molecular Partners, a clinical-stage biopharmaceutical company. The collaboration focused on developing, manufacturing, and commercializing Molecular Partners’ anti-COVID-19 DARPin program, which includes two therapeutic candidates, MP0420 and MP0423. Additionally, the collaboration aimed at leveraging Novartis broad expertise in worldwide drug development, regulatory affairs, manufacturing, and commercialization to quickly advance the program in keeping with the unprecedented global urgency created by the pandemic.
- Aug-2020: Teva Pharmaceutical came into partnership with Alvotech, a fully integrated specialty biopharmaceutical company. This partnership aimed to combine Teva’s long-standing commercial presence & extensive infrastructure in the U.S. market with Alvotech’s scientific experience and state-of-the-art biologics manufacturing.
; Product Launches and Product Expansion:
- May-2022: Biocon Biologics released Abevmy, a biosimilar to Roche’s Avastin. This launch focused on adding another world-class biosimilar to the oncology portfolio in Canada, which includes Ogivri (Trastuzumab) and Fulphila (Pegfilgrastim). Also, Abevmy is expected to allow Biocon to expand patient access to another affordable biologic for cancer care.
- Feb-2021: Fresenius Kabi released IDACIO, an adalimumab biosimilar. The drug is available immediately for all indications of reference medicine in the regions of gastroenterology, rheumatology, and dermatology. As a partner to the Canadian health care system, the company is well known for its high-quality standards & manufacturing capabilities and now for biosimilars, the company invested in comprehensive programs that is expected to make the usage of the product seamless for patients & assist physicians in delivering optimal care.
- Apr-2020: Merck released ONTRUZANT, a biosimilar of the reference biologic medicine Herceptin. ONTRUZANT is available in both 150 mg single-dose vials & 420 mg multiple-dose vials. The launch focused on providing adjuvant treatment of HER2 overexpressing node positive or node negative breast cancer as part of a treatment regimen consisting of cyclophosphamide, doxorubicin, and either paclitaxel or docetaxel.
- Jan-2020: Pfizer launched ZIRABEV, RUXIENCE, & TRAZIMERA, three new biosimilars. The medicines is expected to be launched at the lowest Wholesale Acquisition Cost (WAC) among rituximab, bevacizumab, or trastuzumab products presently available in the market, becoming available at a substantially discounted price to the originator product.
; Acquisitions and Mergers:
- Aug-2022: Amgen completed the acquisition of ChemoCentryx, a biopharmaceutical company focused on orally-administered therapeutics to treat autoimmune diseases, inflammatory disorders, and cancer. This acquisition aimed at enhancing decades-long leadership in inflammation & nephrology with TAVNEOS, transformative, first-in-class treatment for ANCA-associated vasculitis.
- Mar-2022: Fresenius completed the acquisition of mAbxience, a leading international biopharmaceutical company. The acquisition aimed at expanding its business by making cheaper versions of biotechnology drugs that have lost patent protection. Also, the acquisition is expected to further strengthen and leverage Fresenius Kabi's position, as both perfectly complement the company's growth businesses in biopharmaceuticals & medical technology.
- Feb-2022-Feb Biocon Biologics signed an agreement to acquire Viatris, a global pharmaceutical company. The acquisition is expected to create a unique fully integrated, world's leading biosimilars enterprise. The acquisition further is expected to bring together the complementary capabilities & strengths of both partners and prepares the company for the next decade of value creation for all the stakeholders.
; Approvals and Trials:
- Jul-2022: Sandoz got FDA approval for Hyrimoz. The application includes the indications of the refered medicine Humira not protected by orphan exclusivity, which includes rheumatoid arthritis, psoriatic arthritis, juvenile idiopathic arthritis, ulcerative colitis, ankylosing spondylitis, Crohn’s disease, and plaque psoriasis.
- Jun-2022: Amgen got the FDA approval for RIABNI, a biosimilar to Rituxan. This approval is expected to serve as a benefit for adults living with moderate to severe rheumatoid arthritis, a chronic inflammatory joint disease, who now have access to a proven as well as an affordable treatment option.
- May-2022: Teva Pharmaceutical got MHRA approval for ONGAVIA, a ranibizumab biosimilar referencing Genentech’s LUCENTIS. Ranibizumab is expected to inhibit vascular endothelial growth factor (VEGF) that is responsible for the excessive formation of blood vessels in the retina.
- Mar-2022: Fresenius Kabi got approval for European Commission STIMUFEND, a pegfilgrastim biosimilar referencing NEULASTA. STIMUFEND is Fresenius’s first approved molecule in its oncology biosimilar portfolio & second biosimilar approved in Europe. Fresenius also filed its pegfilgrastim biosimilar candidate for regulatory FDA approval and the application is currently under review.
Scope of the Study
Market Segments Covered in the Report:
By Application
- Oncology Diseases
- Blood Disorders
- Chronic & Autoimmune Diseases
- Others
By Type
- Monoclonal Antibodies
- Granulocyte-Colony Stimulating Factor
- Erythropoietin
- Human Growth Hormone
- Insulin
- Others
By Geography
- North America
- US
- Canada
- Mexico
- Rest of North America
- Europe
- Germany
- UK
- France
- Russia
- Spain
- Italy
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- Singapore
- Malaysia
- Rest of Asia Pacific
- LAMEA
- Brazil
- Argentina
- UAE
- Saudi Arabia
- South Africa
- Nigeria
- Rest of LAMEA
Key Market Players
List of Companies Profiled in the Report:
- Amgen, Inc.
- Biocon Limited
- Dr. Reddy’s Laboratories Ltd.
- Eli Lilly and Company
- Intas Pharmaceutical Ltd
- Novartis AG (Sandoz)
- Merck & Co., Inc.
- Pfizer, Inc.
- Teva Pharmaceuticals Industries Ltd.
- Fresenius SE & Co. KGaA
Unique Offerings from the Publisher
- Exhaustive coverage
- The highest number of Market tables and figures
- Subscription-based model available
- Guaranteed best price
- Assured post sales research support with 10% customization free
Table of Contents
Companies Mentioned
- Amgen, Inc.
- Biocon Limited
- Dr. Reddy’s Laboratories Ltd.
- Eli Lilly and Company
- Intas Pharmaceutical Ltd
- Novartis AG (Sandoz)
- Merck & Co., Inc.
- Pfizer, Inc.
- Teva Pharmaceuticals Industries Ltd.
- Fresenius SE & Co. KGaA