Left atrial appendage closure, also called LAA closure or LAAC, is a minimally invasive medical procedure used to drastically reduce the risk of stroke in patients with atrial fibrillation (commonly known as Afib or AF) who have not responded to conventional treatments. By occluding the left atrial appendage with LAA closure devices, the risk of thromboembolism due to LAA is decreased.
If a patient is in danger of forming clots within the LAA/left atrium, the practitioner may consider sealing up the patient's left atrial appendage. This eliminates the need for blood-thinning medicines and decreases the danger of stroke. For the closure of the left atrial appendage, physicians employ a variety of well-known and readily available technologies. Companies across the world are offering revolutionary LAA closure devices to the market.
Blood in the left atrium and LAA is pushed out of the left atrium and into the left ventricle (lower left chamber of the heart) whenever the heart contracts or beats in a healthy individual. In situations of atrial fibrillation, however, the electrical impulses that direct the heartbeat do not occur in the proper motion or sequence. Consequently, several impulses begin at the same moment and are distributed uniformly through the atria. As a result of these incoherencies, blood begins to accumulate and clot. Therefore, when coagulated blood is pumped from the heart, a stroke may follow. Oral anticoagulants lessen the likelihood of having a stroke.
Moreover, LAA closure devices and instruments may be recommended to those who are at risk of acquiring such diseases, with the Watchman Device being one of the most popular on the market. In addition, implanted cardiac devices, like pacemakers or loop recorders, are now utilized to monitor and treat an irregular heartbeat. The increasing frequency of atrial fibrillation and the multiple benefits provided by LAA closure devices are acknowledged as driving drivers for the Left Atrial Appendage Market.
COVID-19 Impact Analysis
The COVID-19 pandemic was an unprecedented public health crisis that had a substantial impact on the left atrial appendage closure device market. The lack of intensive care unit (ICU) beds and anesthesiologists poses a substantial impediment to the performance of non-emergent structural cardiac treatments, such as left atrial appendage closure operations, and the reduction of the usage of left atrial appendage closure devices. Moreover, supply chain disruptions also impeded the distribution of LAA devices all over the world.Market Growth Factors
Increasing Incidences of Atrial Fibrillation All Over the World
One of the major factors that are propelling the growth of the left atrial appendage closure devices market is the increase in the number of people suffering from atrial fibrillation. It is anticipated that the rising incidence of atrial fibrillation would increase the demand for left atrial appendage closure devices to conduct closure surgeries. Atrial fibrillation was one of the most frequent atrial arrhythmias among individuals with congenital cardiac disease all over the world. It also accounts for a significant proportion of mortalities throughout the world.Increased Efficiency and Precision of Endocardial LAA Devices and Percutaneous LAA Devices
The market for endocardial left atrial appendage closure devices is growing at a very rapid rate, which is driven by several advantages along with the higher efficiency of endocardial LAA devices. Endocardial LAA devices are frequently associated with reduced rates of complications and shorter hospital stays than epicardial LAA devices. Multiple clinical trials have demonstrated that endocardial LAA devices provide increased safety and efficacy in stroke treatment and blood loss reduction.Marketing Restraining Factor:
Side Effects Along with The Risk of Infections Linked with LAA Closure Surgery
Atrial fibrillation (AF) is the most prevalent persistent arrhythmia in the world, and its incidence is increasing due to aging and chronic cardiac disease. Anticoagulation is the usual therapy for reducing the risk of stroke in people with nonvalvular AF. In a subgroup of individuals, however, alternative treatment may be recommended due to oral anticoagulation's past failure or the existence of contraindications and limitations to anticoagulation.End-use Outlook
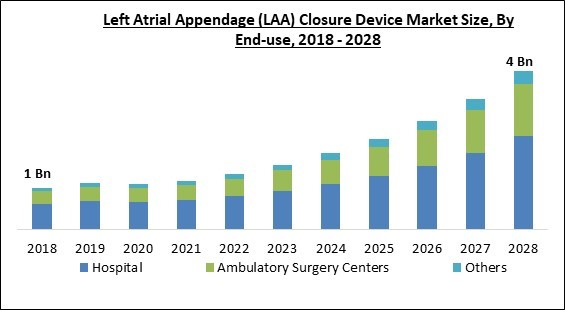
By End-Use, the Left Atrial Appendage (LAA) Closure Device Market is segregated into Hospitals, Ambulatory Surgery Centers, and Others. In 2021, the ambulatory surgery centers segment witnessed a substantial revenue share of the Left Atrial Appendage (LAA) Closure Device Market. While the usage of LAA closure devices is expanding in cardiac clinics and hospitals, their use in ambulatory operations has also expanded due to variables, such as their mobility.Product Outlook
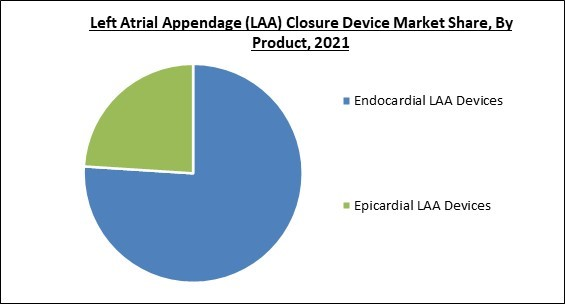
Based on Product, the Left Atrial Appendage (LAA) Closure Device Market is bifurcated into Endocardial LAA Devices and Epicardial LAA Devices. In 2021, the endocardial LAA devices segment acquired the largest revenue share of the Left Atrial Appendage (LAA) Closure Device Market. Patients implanted with endocardial LAA devices had fewer problems, shorter hospital stays, as well as fewer 30-day readmissions. Numerous clinical investigations have demonstrated that endocardial LAA devices are more efficient and safer for controlling stroke and lowering bleeding.Regional Outlook
Region-Wise, the Left Atrial Appendage (LAA) Closure Device Market is analyzed across North America, Europe, Asia-pacific, and LAMEA. North America accounted for the largest revenue share of the Left Atrial Appendage (LAA) Closure Device Market in 2021. Due to a number of variables, including broad acceptance of sophisticated left atrial appendage closure devices, an expansion in the incidence of atrial fibrillation, as well as a robust healthcare infrastructure across regional countries, the prevalence of atrial fibrillation has increased.The market research report covers the analysis of key stakeholders of the market. Key companies profiled in the report include Boston Scientific Corporation, AtriCure, Inc., Abbott Laboratories, Johnson & Johnson (Johnson & Johnson Services, Inc.), Occlutech Holding AG, LifeTech Scientific Corporation, Cardia, Inc. and Lepu Medical Technology Co., Ltd
Scope of the Study
By End-use
- Hospital
- Ambulatory Surgery Centers
- Others
By Product
- Endocardial LAA Devices
- Epicardial LAA Devices
By Geography
- North America
- US
- Canada
- Mexico
- Rest of North America
- Europe
- Germany
- UK
- France
- Russia
- Spain
- Italy
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- Singapore
- Malaysia
- Rest of Asia Pacific
- LAMEA
- Brazil
- Argentina
- UAE
- Saudi Arabia
- South Africa
- Nigeria
- Rest of LAMEA
Key Market Players
List of Companies Profiled in the Report:
- Boston Scientific Corporation
- AtriCure, Inc.
- Abbott Laboratories
- Johnson & Johnson (Johnson & Johnson Services, Inc.)
- Occlutech Holding AG
- LifeTech Scientific Corporation
- Cardia, Inc.
- Lepu Medical Technology Co., Ltd
Unique Offerings
- Exhaustive coverage
- The highest number of market tables and figures
- Subscription-based model available
- Guaranteed best price
- Assured post sales research support with 10% customization free
Table of Contents
Companies Mentioned
- Boston Scientific Corporation
- AtriCure, Inc.
- Abbott Laboratories
- Johnson & Johnson (Johnson & Johnson Services, Inc.)
- Occlutech Holding AG
- LifeTech Scientific Corporation
- Cardia, Inc.
- Lepu Medical Technology Co., Ltd