An extremely uncommon genetic condition called spinal muscular atrophy (SMA) damages the area of the central nervous system that regulates voluntary muscle movements. SMA is a neurological autosomal recessive genetic condition marked by muscular atrophy and deterioration. It is among the most prevalent genetic diseases with devastating symptoms and the main genetic cause of newborn mortality.
SMA is a genetic condition that runs in families. If the child has SMA, it is likely due to the presence of two faulty genes, one from each parent, in their body. Their system won't be capable of producing a particular form of protein as a result, the cells that govern muscles perish without it. If just one of the parents passes on a defective gene to the child, they will not develop SMA but they will be carriers of the condition.
The child might carry the damaged gene onto their offspring when they are adults. Depending on the type, spinal muscular atrophy symptoms might be very different. The most severe symptoms of Type 1 SMA in children are typically present during the initial six months of their lives and are more severe.
Those with milder kinds may not exhibit symptoms until the children are 18 months or older since they are less severe. Scoliosis, respiratory issues, swallowing and issues in feeding, delayed gross motor abilities, decreased muscle tone and muscle weakness, limited mobility, and delayed tongue motions are all possible signs of SMA.
COVID-19 Impact Analysis
Due to severe lockdown procedures and limited human movement, the COVID-19 pandemic has negatively impacted the spinal muscular atrophy treatment market. Since the majority of funding was used for COVID-19 vaccine production, the money obtained for Research and development activities and medication research for SMA was decreasing. Access to care for people with spinal muscular atrophy (SMA) was adversely affected by the pandemic. For instance, Cure SMA, a non-profit organization, voluntary-driven, dedicated solely to eliminating SMA, launched a poll in an attempt to assess the impact of the pandemic on the SMA community.Market Growth Factors
Growing R&D Activities For The Spinal Muscular Atrophy Treatment
The prevalence and knowledge of spinal muscular atrophy have significantly increased, which is driving the demand for effective therapy options that improve patient outcomes. There have been two significant product introductions in the field of gene therapy recently. For a very long time, there was no cure for the rare condition spinal muscular atrophy, which frequently resulted in newborns and young children dying.Increasing Reimbursement Access To Patients
One of the major trends in spinal muscular atrophy (SMA) is expanding the opportunity for the reimbursement of costly medications globally in several countries. This is predicted to have a favorable impact on the uptake of costly gene therapies. France, Germany, Austria, and Italy are a few of the nations that have given all patients access to services that are reimbursed, and Israel and Hong Kong have done the same.Market Restraining Factors
Extremely High Costs Of The Approved Products
The high cost of treating rare genetic illnesses is one of the main factors that has constrained the expansion of the treatment facility. For instance, Spinraza is expected to cost US$ 708,000 in the first year and US$ 354,000 in each year after that, totaling US$ 3.9 million for the expense of treatment over a ten-year period. While a single Novartis therapy for Zolgensma is expected to cost US$ 2.1 million. By comparison, risdiplam can cost up to $340,000 annually. These expenses can undoubtedly cause serious problems for both patients and their families as well as healthcare professionals.Type Outlook
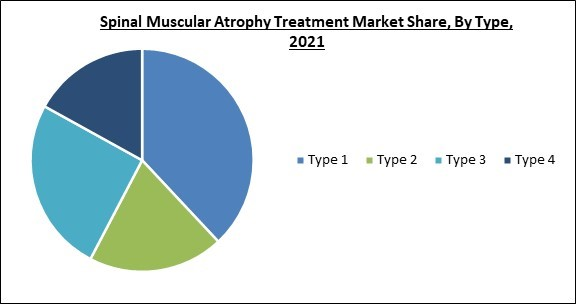
Based on type, the spinal muscular atrophy treatment market is categorized into type 1, type 2, type 3, and type 4. The Type 1 segment garnered the highest revenue share in the spinal muscular atrophy treatment market in 2021. The significant prevalence rate is responsible for this growth. About half of all newborn infants with SMA have this kind of SMA, making it more widespread. A number of treatments, notably Zolgensma (onasemnogene abeparvovec-xioi), have received FDA approval for the treatment of type-1 patients.Treatment Type Outlook
On the basis of treatment type, the spinal muscular atrophy treatment market is divided into gene therapy and drug. The drug segment acquired the largest revenue share in the spinal muscular atrophy treatment market in 2021. The segment is expanding because of the low cost of drug research and development as well as the simplicity of access relative to other treatments. Presently, three major FDA-approved drugs for the treatment of SMA are available on the market.Route Of Administration Outlook
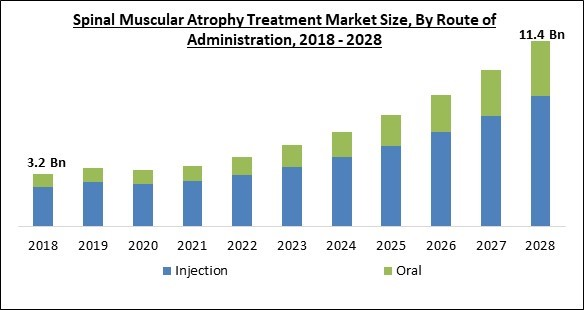
On the basis of route of administration, the spinal muscular atrophy treatment market is classified into oral and injection. The oral segment recorded a substantial revenue share in the spinal muscular atrophy treatment market in 2021. This is due to benefits like non-invasiveness, simplicity of pain avoidance, digestion, adaptability, and fewer side effects as opposed to other methods of administration methods that are linked with it.Regional Outlook
Based on region, the spinal muscular atrophy treatment market is analyzed across North America, Europe, Asia Pacific, and LAMEA. The North America segment procured the largest revenue share in the spinal muscular atrophy treatment market in 2021. The key drivers of the region's domination are the increasing incidence of SMA conditions and rising spending on treatment supplies. For instance, between 10,000 and 25,000 youngsters and adults reside in the United States who have SMA.The market research report covers the analysis of key stakeholders of the market. Key companies profiled in the report include Biogen, Inc., Novartis AG, Ionis Pharmaceuticals, Inc., PTC Therapeutics, Inc., NMD Pharma A/S, Scholar Rock, Inc. (Scholar Rock Holding Corporation), Cytokinetics, Inc., Biohaven Pharmaceutical Holding Company Ltd. (Pfizer, Inc.), Astellas Pharma, Inc., and F. Hoffmann-La Roche Ltd. (Genentech, Inc.)
Strategies Deployed in Spinal Muscular Atrophy Treatment Market
- May-2022: Genentech received approval from FDA for Evrysdi (risdiplam) for use in babies under two months with SMA. Evrysdi (risdiplam) is an early treatment for SMA and can be taken at home.
- Feb-2022: Biohaven signed an agreement with Bristol Myers Squibb, a US-based pharmaceutical company primarily into developing, and discovering therapeutics. The agreement involves transferring the development and commercialization rights of taldefgrobep alfa, a phase-3 ready anti-myostatin adnectin. This transfer of rights broadened Biohaven's product portfolio of psychiatric, neuroinflammatory, and neurologic treatments.
- Mar-2021: PTC Therapeutics got approval from European Commission for Evrysdi, a liquid dose, developed to treat Spinal Muscular Atrophy, which can be taken at home, and can be taken by both children and adults.
- Mar-2021: PTC extended its partnership with Spinal Muscular Atrophy (SMA) Foundation, a non-profit organization focused on developing treatments for SMA. The expansion aims to reinforce drug discovery and development research focused on regenerative medicine.
- Oct-2020: Ionis completed the acquisition of Akcea Therapeutics, a biopharmaceutical firm engaged in developing and marketing therapies and medicines focused on treating patients with rare diseases. This acquisition enables Ionis to generate value from Akcea's underway therapies and products.
- Jun-2020: PTC took over Censa Pharmaceuticals, a biopharmaceutical company primarily into developing CNSA-001 (sepiapterin), a pharmacological treatment meant for orphan metabolic diseases. This acquisition reinforced PTC's existing rare disease portfolio.
- May-2020: NMD Pharma expanded its geographical footprint by establishing a new subsidiary, NMD Pharma US, Inc. in the United States. This expansion aligns with NMD's growth strategy and enables them to further reinforce and expand their pipeline in rare neuromuscular diseases.
- Mar-2020: Novartis received approval for Zolgensma from the Japanese Ministry of Health, Labour, and Welfare. Zolgensma is a gene therapy for patients below the age of two years suffering from spinal muscular atrophy. A single dose of Zolgensma is capable of making a significant positive impact on the patient's condition.
- Nov-2018: Genentech acquired Jecure Therapeutics, a biotech company focused on developing drugs for inflammatory diseases. The acquisition complemented Genentech's proficiency in NLRP3 biology and enabled them to better take care of patients with inflammatory diseases.
- Aug-2018: PTC acquired Agilis Biotherapeutics, a biotechnology company primarily developing DNA therapeutics focused on rare diseases. This acquisition lines up with PTC's aim of being a leader in rare disorders treatment.
- May-2018: Novartis acquired AveXis, a biotechnology company providing treatment services for rare neurological genetic disorders. This acquisition aligns with Novartis's plan to provide life-changing innovation for unmet medical requirements and further complements its expanding pipeline of gene therapies focused on the treatment of diseases ranging from SMA to blindness.
Scope of the Study
By Type
- Type 1
- Type 2
- Type 3
- Type 4
By Route of Administration
- Injection
- Oral
By Treatment Type
- Drug
- Spinraza
- Zolgensma (AVXS-101)
- Evrysdi
- Others
- Gene Therapy
By Geography
- North America
- US
- Canada
- Mexico
- Rest of North America
- Europe
- Germany
- UK
- France
- Russia
- Spain
- Italy
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- Australia
- Malaysia
- Rest of Asia Pacific
- LAMEA
- Brazil
- Argentina
- UAE
- Saudi Arabia
- South Africa
- Nigeria
- Rest of LAMEA
Key Market Players
List of Companies Profiled in the Report:
- Biogen, Inc.
- Novartis AG
- Ionis Pharmaceuticals, Inc.
- PTC Therapeutics, Inc.
- NMD Pharma A/S
- Scholar Rock, Inc. (Scholar Rock Holding Corporation)
- Cytokinetics, Inc.
- Biohaven Pharmaceutical Holding Company Ltd. (Pfizer, Inc.)
- Astellas Pharma, Inc.
- F.Hoffmann-La Roche Ltd. (Genentech, Inc.)
Unique Offerings
- Exhaustive coverage
- The highest number of Market tables and figures
- Subscription-based model available
- Guaranteed best price
- Assured post sales research support with 10% customization free
Table of Contents
Companies Mentioned
- Biogen, Inc.
- Novartis AG
- Ionis Pharmaceuticals, Inc.
- PTC Therapeutics, Inc.
- NMD Pharma A/S
- Scholar Rock, Inc. (Scholar Rock Holding Corporation)
- Cytokinetics, Inc.
- Biohaven Pharmaceutical Holding Company Ltd. (Pfizer, Inc.)
- Astellas Pharma, Inc.
- F. Hoffmann-La Roche Ltd. (Genentech, Inc.)