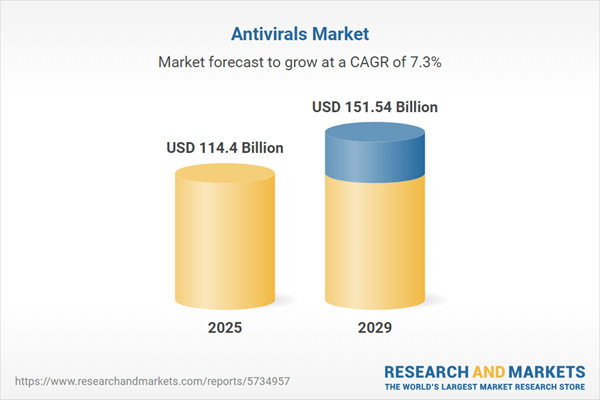
The antivirals market size is expected to see strong growth in the next few years. It will grow to $151.54 billion in 2029 at a compound annual growth rate (CAGR) of 7.3%. The growth in the forecast period can be attributed to emerging viral threats, global health initiatives. Major trends in the forecast period include technological advances, telemedicine and digital health, personalized medicine.
The forecast of 7.3% growth over the next five years reflects a modest reduction of 0.3% from the previous estimate for this market. This reduction is primarily due to the impact of tariffs between the US and other countries. Trade tensions could hinder U.S. pandemic preparedness by inflating prices of neuraminidase inhibitors like oseltamivir manufactured in Switzerland and India, resulting in delayed influenza treatment and higher seasonal outbreak response expenditures. The effect will also be felt more widely due to reciprocal tariffs and the negative effect on the global economy and trade due to increased trade tensions and restrictions.
The rising incidence of influenza is expected to drive the growth of the antiviral market. Influenza, commonly known as the flu, is a highly contagious viral respiratory infection caused by influenza viruses. Antiviral medications are used to treat influenza by inhibiting the replication and spread of these viruses within the body. For instance, in October 2022, the Centers for Disease Control and Prevention (CDC), a US-based health agency, reported that during the 2021-2022 flu season, there were 9 million flu illnesses, 4 million flu-related medical visits, 100,000 flu-related hospitalizations, and 5,000 flu deaths. This increasing incidence of influenza is a key factor contributing to the growth of the antiviral market.
The growing public-private funding for life science research globally is expected to accelerate the growth of the antiviral drug therapy market. While no drugs or vaccines are currently approved for the treatment of COVID-19, several vaccines and therapies are in the pipeline awaiting approval or launch. Governments worldwide have introduced new grant funds to support research in vaccines, treatments, and diagnostics. For example, in March 2023, Gov.UK, a UK-based government agency, alongside four UK life sciences companies, received the first tranche of £277 million ($305.78 million) from the Life Sciences Innovative Manufacturing Fund. This initiative is expected to facilitate an additional £260 million ($287.01 million) in private investment, create 320 new jobs, and safeguard 199 existing ones. This follows a previous pilot fund of £75 million ($82.79 million), which secured 224 new jobs and protected 345 positions in the sector. As such, the increase in public-private funding for life science research is anticipated to boost research and development efforts, thereby driving the growth of the COVID-19 antiviral drug therapy market.
Major companies operating in the antiviral market are focusing on developing innovative treatments, such as oral antiviral medications, to improve patient compliance, enhance accessibility, reduce healthcare costs, and provide more convenient and effective options for managing viral infections. Oral antiviral treatments are taken by mouth and work by inhibiting the replication or spread of viruses in the body, helping to reduce the severity and duration of infections. For example, in May 2023, Pfizer, a US-based pharmaceutical company, announced the approval of Paxlovid by the Food and Drug Administration (FDA). This marked the first oral antiviral treatment for COVID-19 in adults. The approval is significant as it provides a new therapeutic option for managing COVID-19, especially as the pandemic transitions to an endemic phase. Paxlovid is indicated for adults with mild-to-moderate COVID-19 who are at high risk of progression to severe disease, including hospitalization or death. This treatment is expected to improve patient outcomes while offering a more convenient, oral option compared to intravenous treatments.
Major companies in the antiviral market are increasingly focused on developing advanced therapeutic solutions, such as antiviral treatments, to improve patient outcomes and reduce viral transmission. These treatments are crucial for managing viral infections and preventing complications in affected patients. For example, in August 2024, Red Queen’s Technology, a US-based pharmaceutical company, launched a new line of versatile antivirals. Apple Tree Partners invested $55 million in Red Queen Therapeutics, a biotech firm working on broad-spectrum antiviral treatments. The company targets infectious pathogens such as COVID-19 and influenza, having already advanced a COVID antiviral through Phase 1 testing and secured U.S. government funding for a pan-influenza drug. Co-founded by Harvard professor Loren Walensky, Red Queen uses stapled lipopeptides to inhibit viral fusion and block viral entry into cells.
In June 2022, Pfizer Inc., a leading US-based pharmaceutical company, completed its acquisition of ReViral Ltd. for $525 million. This strategic acquisition is part of Pfizer's effort to expand its anti-infective pipeline and strengthen its dedication to developing medications and vaccines for combating respiratory syncytial virus (RSV). ReViral Ltd., a UK-based company, specializes in the development of antiviral drugs. This acquisition enhances Pfizer's position in the fight against viral infections, particularly RSV, and represents a significant investment in the development of antiviral treatments.
Major companies operating in the antivirals market include AbbVie Inc., Bristol-Myers-Squibb Co., Chemical Industrial & Pharmaceutical Laboratories Ltd., F. Hoffmann-La Roche Ltd., Gilead Sciences Inc., GlaxoSmithKline Plc, Johnson & Johnson Services Ltd., Merck & Co. Inc., Dr. Reddy’s Laboratories Ltd., AstraZeneca plc, Aurobindo Pharma Ltd., Abbott Laboratories, Schering-Plough Corporation, Pfizer Inc., Sanofi-Synthélabo Ltd., Regeneron Pharmaceuticals Inc., Inovio Pharmaceuticals Inc., Novavax Inc., BioCryst Pharmaceuticals Inc., Alnylam Pharmaceuticals Inc., Argos Distributors Limited, AVI Biopharma International Ltd., Moderna Inc., BioNTech SE, Eli Lilly and Company, Takeda Pharmaceutical Company Limited, Biogen Inc., Genentech USA Inc., Vertex Pharmaceuticals Inc.
North America was the largest region in the anti-viral drug therapy market in 2024. The Middle East is expected to be the fastest-growing region in the antivirals market during the forecast period. The regions covered in the antivirals market report are Asia-Pacific, Western Europe, Eastern Europe, North America, South America, Middle East, Africa. The countries covered in the antivirals market report are Australia, Brazil, China, France, Germany, India, Indonesia, Japan, Russia, South Korea, UK, USA, Canada, Italy, Spain.
Note that the outlook for this market is being affected by rapid changes in trade relations and tariffs globally. The report will be updated prior to delivery to reflect the latest status, including revised forecasts and quantified impact analysis. The report’s Recommendations and Conclusions sections will be updated to give strategies for entities dealing with the fast-moving international environment.
The sudden escalation of U.S. tariffs and the resulting trade tensions in spring 2025 are having a significant impact on the pharmaceutical sector. Companies are grappling with higher costs on imported active pharmaceutical ingredients (APIs), glass vials, and laboratory equipment - many of which have limited alternative sources. Generic drug manufacturers, already operating with minimal profit margins, are particularly affected, with some scaling back production of low-margin medications. Biotech firms are also experiencing delays in clinical trials due to shortages of specialized reagents linked to tariffs. In response, the industry is shifting API production to regions like India and Europe, building up inventory reserves, and advocating for tariff exemptions on essential medicines.
The anti-viral drug therapy market research report is one of a series of new reports that provides anti-viral drug therapy market statistics, including anti-viral drug therapy industry global market size, regional shares, competitors with an anti-viral drug therapy market share, detailed anti-viral drug therapy market segments, market trends and opportunities, and any further data you may need to thrive in the anti-viral drug therapy industry. This anti-viral drug therapy market research report delivers a complete perspective of everything you need, with an in-depth analysis of the current and future scenario of the industry.
Anti-viral drug therapy encompasses the use of medications to combat viral infections, including diseases like human immunodeficiency virus (HIV), hepatitis, influenza, and emerging viruses like the novel COVID-19. These drugs work by inhibiting the replication and spread of viruses rather than directly killing them. The anti-viral drug sector primarily comprises establishments engaged in the production of various classes of antiviral drugs, including DNA polymerase inhibitors, reverse transcriptase inhibitors, protease inhibitors, neuraminidase inhibitors, and others.
The primary categories of anti-viral drug therapy include branded and generic medications. These drugs belong to different classes tailored for specific viral infections, including HIV, hepatitis, herpes, influenza, and more. Among these, reverse-transcriptase inhibitors (RTIs) are a noteworthy class used for treating HIV and, in some cases, hepatitis B infections.
The anti-viral drug therapy market consists of sales of amantadine and rimantadine. Values in this market are factory gate values, that is the value of goods sold by the manufacturers or creators of the goods, whether to other entities (including downstream manufacturers, wholesalers, distributors and retailers) or directly to end customers. The value of goods in this market includes related services sold by the creators of the goods.
The market value is defined as the revenues that enterprises gain from the sale of goods and/or services within the specified market and geography through sales, grants, or donations in terms of the currency (in USD, unless otherwise specified).
The revenues for a specified geography are consumption values that are revenues generated by organizations in the specified geography within the market, irrespective of where they are produced. It does not include revenues from resales along the supply chain, either further along the supply chain or as part of other products.
This product will be delivered within 1-3 business days.
Table of Contents
Executive Summary
Antivirals Global Market Report 2025 provides strategists, marketers and senior management with the critical information they need to assess the market.This report focuses on antivirals market which is experiencing strong growth. The report gives a guide to the trends which will be shaping the market over the next ten years and beyond.
Reasons to Purchase:
- Gain a truly global perspective with the most comprehensive report available on this market covering 15 geographies.
- Assess the impact of key macro factors such as geopolitical conflicts, trade policies and tariffs, post-pandemic supply chain realignment, inflation and interest rate fluctuations, and evolving regulatory landscapes.
- Create regional and country strategies on the basis of local data and analysis.
- Identify growth segments for investment.
- Outperform competitors using forecast data and the drivers and trends shaping the market.
- Understand customers based on the latest market shares.
- Benchmark performance against key competitors.
- Suitable for supporting your internal and external presentations with reliable high quality data and analysis
- Report will be updated with the latest data and delivered to you along with an Excel data sheet for easy data extraction and analysis.
- All data from the report will also be delivered in an excel dashboard format.
Description
Where is the largest and fastest growing market for antivirals? How does the market relate to the overall economy, demography and other similar markets? What forces will shape the market going forward, including technological disruption, regulatory shifts, and changing consumer preferences? The antivirals market global report answers all these questions and many more.The report covers market characteristics, size and growth, segmentation, regional and country breakdowns, competitive landscape, market shares, trends and strategies for this market. It traces the market’s historic and forecast market growth by geography.
- The market characteristics section of the report defines and explains the market.
- The market size section gives the market size ($b) covering both the historic growth of the market, and forecasting its development.
- The forecasts are made after considering the major factors currently impacting the market. These include: the technological advancements such as AI and automation, Russia-Ukraine war, trade tariffs (government-imposed import/export duties), elevated inflation and interest rates.
- Market segmentations break down the market into sub markets.
- The regional and country breakdowns section gives an analysis of the market in each geography and the size of the market by geography and compares their historic and forecast growth.
- The competitive landscape chapter gives a description of the competitive nature of the market, market shares, and a description of the leading companies. Key financial deals which have shaped the market in recent years are identified.
- The trends and strategies section analyses the shape of the market as it emerges from the crisis and suggests how companies can grow as the market recovers.
Scope
Markets Covered:
1) By Type: Branded; Generic2) By Drug Class: DNA Polymerase Inhibitors; Reverse Transcriptase Inhibitors; Protease Inhibitors; Neuraminidase Inhibitors; Other Drug classes
3) By Application: HIV; Hepatitis; Herpes; Influenza; Other Applications
Subsegments:
1) By Branded: Antivirals for HIV or AIDS; Antivirals for Hepatitis; Antivirals for Influenza; Antivirals for Herpes Simplex Virus (HSV)2) By Generic: Generic HIV or AIDS Antivirals; Generic Hepatitis Antivirals; Generic Influenza Antivirals; Generic Herpes Simplex Virus (HSV) Antivirals
Companies Mentioned: AbbVie Inc.; Bristol-Myers-Squibb Co.; Chemical Industrial & Pharmaceutical Laboratories Ltd.; F. Hoffmann-La Roche Ltd.; Gilead Sciences Inc.; GlaxoSmithKline Plc; Johnson & Johnson Services Ltd.; Merck & Co. Inc.; Dr. Reddy’s Laboratories Ltd.; AstraZeneca plc; Aurobindo Pharma Ltd.; Abbott Laboratories; Schering-Plough Corporation; Pfizer Inc.; Sanofi-Synthélabo Ltd.; Regeneron Pharmaceuticals Inc.; Inovio Pharmaceuticals Inc.; Novavax Inc.; BioCryst Pharmaceuticals Inc.; Alnylam Pharmaceuticals Inc.; Argos Distributors Limited; AVI Biopharma International Ltd.; Moderna Inc.; BioNTech SE; Eli Lilly and Company; Takeda Pharmaceutical Company Limited; Biogen Inc.; Genentech USA Inc.; Vertex Pharmaceuticals Inc.
Countries: Australia; Brazil; China; France; Germany; India; Indonesia; Japan; Russia; South Korea; UK; USA; Canada; Italy; Spain
Regions: Asia-Pacific; Western Europe; Eastern Europe; North America; South America; Middle East; Africa
Time Series: Five years historic and ten years forecast.
Data: Ratios of market size and growth to related markets, GDP proportions, expenditure per capita.
Data Segmentation: Country and regional historic and forecast data, market share of competitors, market segments.
Sourcing and Referencing: Data and analysis throughout the report is sourced using end notes.
Delivery Format: PDF, Word and Excel Data Dashboard.
Companies Mentioned
The companies featured in this Antivirals market report include:- AbbVie Inc.
- Bristol-Myers-Squibb Co.
- Chemical Industrial & Pharmaceutical Laboratories Ltd.
- F. Hoffmann-La Roche Ltd.
- Gilead Sciences Inc.
- GlaxoSmithKline Plc
- Johnson & Johnson Services Ltd.
- Merck & Co. Inc.
- Dr. Reddy’s Laboratories Ltd.
- AstraZeneca plc
- Aurobindo Pharma Ltd.
- Abbott Laboratories
- Schering-Plough Corporation
- Pfizer Inc.
- Sanofi-Synthélabo Ltd.
- Regeneron Pharmaceuticals Inc.
- Inovio Pharmaceuticals Inc.
- Novavax Inc.
- BioCryst Pharmaceuticals Inc.
- Alnylam Pharmaceuticals Inc.
- Argos Distributors Limited
- AVI Biopharma International Ltd.
- Moderna Inc.
- BioNTech SE
- Eli Lilly and Company
- Takeda Pharmaceutical Company Limited
- Biogen Inc.
- Genentech USA Inc.
- Vertex Pharmaceuticals Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 250 |
| Published | September 2025 |
| Forecast Period | 2025 - 2029 |
| Estimated Market Value ( USD | $ 114.4 Billion |
| Forecasted Market Value ( USD | $ 151.54 Billion |
| Compound Annual Growth Rate | 7.3% |
| Regions Covered | Global |
| No. of Companies Mentioned | 30 |