CELL THERAPY MEDIA MARKET: GROWTH AND TRENDS
Fueled by significant advancements and the established efficacy of FDA-approved cell therapies for treating cancer, rare diseases, and chronic conditions, the cell therapy media market has garnered significant interest from stakeholders in the healthcare sector. Interestingly, over 1,000 clinical trials primarily focused on cell therapies have been registered since 2019. Further, more than 35 cell and gene therapies have been brought to market in different regions around the world. Recent cell therapy approval includes Breyanzi®, Carvykti™, and Abecma®.With rising regulatory stringency associated with producing consumables for cell therapy, more than 90% of developers in this field opt to outsource culture media, kits, reagents, and extracellular matrices to suppliers with the required expertise to provide high-quality raw materials. Currently, over 80 companies are providing more than 450 research and / or therapeutic grade raw materials. Moreover, certain companies claim to have GMP-certified facilities for manufacturing consumables intended for various human cells, such as T-cells, stem cells, dendritic cells, and NK cells.
With the ongoing evolution of cell therapy, we expect a rise in innovation and partnerships in the industry, leading to new therapeutic alternatives with the potential to revolutionize patient care and broaden the scope of regenerative medicine.
CELL THERAPY MEDIA MARKET: KEY INSIGHTS
The report delves into the current state of the cell therapy media market and identifies potential growth opportunities within the industry. Some key findings from the report include:- Leveraging their expertise, over 450 types of kits, media, reagents and extracellular matrices have been manufactured by consumable providers for research and therapeutic purposes.
- The market features the presence of over 80 firms across the globe; the majority of these stakeholders are emerging players based in North America.
- A larger proportion of the kits available in the cell therapy domain comprises different types of reagents; of these, 60% are intended for use with stem cell therapies and nearly 80% are stored in refrigerated conditions.
- Media developers are offering products for a broad range of cell therapies; nearly 90% of such players are providing media in the volume range of 100 to 500 ml.
- Predominantly, the cell therapy reagents are intended to be used at the discovery scale of operation for a wide spectrum of functions, including cell expansion and proliferation.
- The market landscape of matrices, which typically have a shelf life of 1 to 1.5 years, is well distributed in terms of type of ECM coating and type of formulation.
- In pursuit of gaining a competitive edge, cell therapy consumable providers are upgrading their existing technologies and expanding their product portfolios.
- Many companies have undertaken strategic initiatives, including partnerships, acquisitions and expansions, to augment their existing capabilities.
- We expect industry stakeholders to continue to forge strategic alliances with niche / specialized players engaged in this domain to further augment their respective product offerings.
- Over the years, stakeholders within this industry have established strong brand positions by undertaking a range of initiatives to further advance the development of raw materials for cell therapies.
- Cost is a key determinant for the adoption of consumables in a cell therapy manufacturing process.
- In 2035, the commercial scale of operation is likely to account for 75% of the total demand for cell therapy consumables; this is attributed to the expected surge in the anticipated approvals of multiple cell therapies.
- A paradigm shift from animal-based to animal component free formulations, combined with stringent regulatory guidelines, is likely to drive the growth of the cell therapy consumables market at an annualized rate of 11.1%.
CELL THERAPY MEDIA MARKET: KEY SEGMENTS
Extracellular Matrices is the Fastest Growing Segment in the Cell Therapy Media Market
Based on the type of product, the market is segmented into culture media, kits, cell culture reagents and extracellular matrices. It is worth highlighting that majority of the current cell therapy media market is captured by culture media.T-Cell Therapy is Likely to Dominate the Cell Therapy Media Market During the Forecast Period
Based on the type of cell therapy, the market is segmented into T-Cell therapy, stem cell therapy, dendritic cell therapy and NK cell therapy. It is worth highlighting that the cell therapy consumables market for NK cell therapies is likely to grow at a relatively higher CAGR, during the forecast period. This can be attributed to the fact that currently, more than 75 NK cell therapy focused clinical studies are being evaluated for a myriad of disease indications. In the coming years, this number is anticipated to increase further, and this is likely to boost the market opportunity for such therapies.By Scale of Operation, Commercial Scale is Likely to Dominate the Cell Therapy Media Market During the Forecast Period
Based on the scale of operation, the market is segmented into clinical and commercial scales. It is worth highlighting that the commercial scale cell therapy media market is likely to drive the market in the near future.Industry Players Segment Accounts for the Largest Share of the Cell Therapy Media Market
Based on the type of end user, the market is segmented into industry and non-industry. It is worth highlighting that the cell therapy media market for industry players is likely to drive the market in the near future. This can be attributed to the fact that the industry players contribute significantly to the overall commercial market as majority of the approved therapies have been developed by such players.North America Accounts for the Largest Share of the Market
Based on key geographical regions, the market is segmented into North America, Europe, Asia-Pacific, Middle East and North Africa, and Latin America. It is worth highlighting that, over the years, the market in Latin America is expected to grow at a higher CAGR.Example Players in the Cell Therapy Media Market
- BD Biosciences
- Bio-Techne
- CellGenix
- Corning
- Irvine Scientific (Acquired by FUJIFILM)
- Lonza
- Miltenyi Biotech
- Sartorius
- STEMCELL Technologies
- Thermo Fisher Scientific
Primary Research Overview
The opinions and insights presented in this study were influenced by discussions conducted with multiple stakeholders. The research report features detailed transcripts of interviews held with the following industry stakeholders:- Vice President of Business Development, Company A
- Chief Operating Officer, Company B
- Director R&D, Cell Culture and Immunology, Company C
- Assistant R&D Manager, Animal Cell Culture, Company D
CELL THERAPY MEDIA MARKET: RESEARCH COVERAGE
- Market Forecast and Opportunity Analysis: The report features an in-depth analysis of the cell therapy media market, focusing on key market segments, including [A] type of product, [B] type of cell therapy, [C] scale of operation, [D] type of end user and [E] key geographical regions.
- Market Landscape: A comprehensive evaluation of companies offering cell culture consumables and cell culture media, considering various parameters, [A] such as year of establishment, [B] company size (in terms of number of employees), [C] location of headquarters, [D] type of product, [E] number and location of consumable facilities, [F] accreditations received, [G] type of end-user, [H] cell culture media compatibility, [I] type of cell therapy, [J] type of function, [K] kit components, [L] type of ECM coating, [M] type of formulation, [N] shelf life, [O] scale of operation, [P] application area, and [Q] storage temperature.
- Company Competitiveness Analysis: A comprehensive competitive analysis of companies operating in the cell culture consumable and cell culture media market, examining factors such as [A] supplier strength, [B] portfolio strength and [C] number of products offered.
- Brand Positioning Analysis: A detailed brand positioning analysis of prominent industry players, highlighting the current perceptions regarding their proprietary brands across different consumable classes.
- Company Profiles: In-depth profiles of key industry players in cell therapy consumables and cell culture media market, focusing on [A] company overviews, [B] product portfolio, [C] consumable facilities, [D] recent developments and an [E] informed future outlook.
- Recent Developments and Initiatives: An analysis of recent developments within the cell culture consumables and cell culture media market, [A] covering partnerships and collaborations, [B] mergers and acquisitions, and [B] expansion initiatives.
- Likely Partner Analysis: A detailed evaluation of over 250 cell therapy developers that are most likely to collaborate with cell culture consumables and cell culture media providers. This analysis considers various relevant parameters, including [A] developer strength (which takes into account a company’s size and its experience in this field), [B] pipeline strength and [C] maturity (based on the number of pipeline drugs and affiliated stage of development), and availability of other cell therapy capabilities.
- Roots Analysis Pricing Strategy: A proprietary Roots Analysis competitive pricing framework, which analyzes the competitive position of various companies engaged in cell culture consumables and cell culture media market, by taking into consideration the prices and features of their consumable offerings (such as media and extracellular matrices).
- Demand Analysis: Informed estimates of the annual demand for cell culture consumables and cell culture media (in terms of volume of media required for total number of cells), based on [A] scale of operation and [B] key geographical regions.
- Upcoming Trends And Future Growth Opportunities: A comprehensive analysis of emerging trends and future growth prospects in the cell culture consumables and media market. It includes details related to the significance of automation in cell therapy manufacturing processes and the benefits of single use technologies for the production of cell therapies.
KEY QUESTIONS ANSWERED IN THIS REPORT
- What are the factors driving the cell therapy consumables market?
- How many players are engaged in offering cell culture media for manufacturing cell therapies?
- How many players are engaged in offering kits for manufacturing cell therapies?
- How many media products are available in the market for culturing cell therapies?
- What are the partnership and collaboration trends observed in the cell therapy consumables domain?
- Which geographical segment captures the largest market share in the current cell therapy consumables market?
- Which type of product contributes to the largest share of the cell therapy consumables market?
REASONS TO BUY THIS REPORT
- The report provides a comprehensive market analysis, offering detailed revenue projections of the overall market and its specific sub-segments. This information is valuable to both established market leaders and emerging entrants.
- Stakeholders can leverage the report to gain a deeper understanding of the competitive dynamics within the market. By analyzing the competitive landscape, businesses can make informed decisions to optimize their market positioning and develop effective go-to-market strategies.
- The report offers stakeholders a comprehensive overview of the market, including key drivers, barriers, opportunities, and challenges. This information empowers stakeholders to stay abreast of market trends and make data-driven decisions to capitalize on growth prospects.
ADDITIONAL BENEFITS
- Complimentary PPT Insights Packs
- Complimentary Excel Data Packs for all Analytical Modules in the Report
- 15% Free Content Customization
- Detailed Report Walkthrough Session with the Research Team
- Free Updated report if the report is 6-12 months old or older
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Accellta
- Acer Therapeutics
- ACROBiosystems
- Activartis Biotech
- AddLife
- Adicet Bio
- Aduro Biotech
- Advanced BioMatrix (Acquired by BICO)
- Advent Bioservices
- AGC Biologics
- AgenTus Therapeutics
- Agilent Technologies
- Ajinomoto
- Akadeum Life Sciences
- Akron Biotech
- Allele Biotechnology and Pharmaceuticals
- Allife Medical Science and Technology
- Allogene Therapeutics
- Altor BioScience
- American CryoStem
- Amphera
- AMSBIO
- ANI Pharmaceuticals
- apceth Biopharma
- Applied Biological Materials
- Applied Cells
- Arbele
- Arcline Investment Management
- Argos Therapeutics
- Aspire Health Science
- Asterias Biotherapeutics
- ATCC
- Athersys
- Atlantis Bioscience
- Atreca
- Aurora Biopharma
- Austrianova
- Autolus
- Avantor
- AVAX Technologies
- Axion BioSystems
- Axol Bioscience
- Basic Pharma
- BD Biosciences
- Beijing Biohealthcare Biotechnology
- Beijing Doing Biomedical
- Beijing Immunochina Medical Science & Technology
- Beijing Sanwater Biological Technology
- Beijing Tricision Biotherapeutics
- Bellicum Pharmaceuticals
- Bio Elpida
- BioAtla
- BioCardia
- BioCare
- BioCentriq
- Bio-Connect
- BioInno Bioscience
- Bioinova
- Biological Industries
- BioRestorative Therapies
- Bio-Techne
- Biowest
- Bone Therapeutics
- BrainStorm Cell Therapeutics
- Brigham and Women’s Hospital
- C3i (Center of Excellence for Cellular Therapy)
- Caladrius Biosciences
- Capricor Therapeutics
- Captain T Cell
- Captivate Bio
- CardioCell
- Carina Biotech
- CARsgen Therapeutics
- CAR-T (Shanghai) Biotechnology
- Cartherics
- Celixir
- Cell and Gene Therapy Catapult
- Cell Applications
- Cell Biologics
- Cell Biotech
- Cell Culture Technologies
- Cell Systems
- Cell Therapies
- Cell-Easy
- Cellectis
- CellGenix
- Cellin Technologies
- Cellmed
- CellMP (A subsidiary of Emmecell)
- CELLnTEC Advanced Cell Systems
- CellPraxis
- CellProtect Nordic Pharmaceuticals
- CellProthera
- Cells for Cells
- Cells Online
- CellSystems
- Cellular Biomedicine Group
- Cellular Engineering Technologies
- Cellular Therapeutics
- Celprogen
- Celularity
- Celyad
- Censo Biotechnologies
- CHA Biolab
- Chengdu MedGenCell
- CiMaas
- Cincinnati Children’s Hospital Medical Center
- Clean Cells
- CO.DON
- Cogent Biosciences
- Cognate BioServices
- CoImmune
- CollPlant
- Cook MyoSite
- Corestem
- Corning
- Creative Bioarray
- Creative Biolabs
- CRISPR Therapeutics
- Cyagen
- Cytiva
- Cytopeutics
- CytoSen Therapeutics (Acquired by Kiadis Pharma)
- Cytovac
- CyTuVax
- DanDrit Biotech
- DCPrime
- Delta-Vir
- denovoMATRIX
- DiscGenics
- ElevateBio
- Endocyte
- Eureka Therapeutics
- Eutilex
- EXUMA Biotech
- Fate Therapeutics
- Fibrocell Technologies
- Five Prime Therapeutics
- Formula Pharmaceuticals
- Fortress Biotech
- Froceth
- FroggaBio
- FUJIFILM Cellular Dynamics
- FUJIFILM Wako Pure Chemicals
- Gadeta
- Gamida Cell
- GE Healthcare
- GeminiBio
- GenCure
- GenCure (A subsidiary of BioBridge Global)
- GenIbet Biopharmaceuticals
- GigaGen
- Global Cell Med
- Glycostem Therapeutics
- Gradalis
- GRI Bio
- Guangzhou Trinomab Biotech
- Heat Biologics
- Hebei Senlang Biotechnology
- Hemostemix
- HengRui YuanZheng Bio-Technology
- HiMedia Laboratories
- Histocell
- Histogenics
- Hitachi Chemical
- Holostem Terapie Avanzate
- Hunan Zhaotai Yongren Biotech
- IBA Lifesciences
- iCarTAB BioMed
- iCell Gene Therapeutics
- Immatics
- Immune Therapeutics
- Immunicum
- ImmunoCellular Therapeutics
- Immunovative Therapies
- Immutep
- IncoCell Tianjin (A subsidiary of Boyalife)
- Incysus
- Innoprot
- Innovative Cellular Therapeutics
- inRegen
- International Stem Cell Corporation
- InVitria
- Iovance Biotherapeutics
- Irvine Scientific (Acquired by FUJIFILM)
- ISTO Technologies
- Ivy Life Sciences
- iXCells Biotechnologies
- Japan Tissue Engineering
- JSR
- JW Biotechnology
- JW CreaGene
- Kadimastem
- Kangstem Biotech
- Kiadis Pharma
- Kiromic
- Kuur Therapeutics
- LAgen Laboratories
- Laurus Labs
- Laurus Bio
- Leucid Bio
- Life Technologies
- Lifecells
- Lifeline Cell Technology
- Lineage Cell Therapeutics
- Lion TCR
- Living Pharma
- Longeveron
- Lonza
- Lumos Pharma
- Lyell Immunopharma
- Lykan Bioscience
- Marino Biotechnology
- Matrixome
- MaxCyte
- MBL Beijing Biotech
- Medeor Therapeutics
- MEDianus Pharma
- Medigene
- MEDINET
- MedVax Technologies
- Mesoblast
- Metrion Biosciences
- Miltenyi Biotech
- Minerva Biotechnologies
- Minovia Therapeutics
- MolecuVax
- Moraga Biotechnology
- MultiClonal Therapeutics
- Multimmune
- Multus Biotechnology
- Mustang Bio
- Nanjing KAEDI Biotechnology
- NantKwest
- NeuCyte
- Neuralstem
- Neuromics
- Nikon CeLL innovation
- Nkarta
- Nohla Therapeutics
- Noile-Immune Biotech
- Northern Therapeutics
- Northwest Biotherapeutics
- Novadip Biosciences
- NovaRx (Acquired by Viropro)
- Novex Innovations
- Nucleus Biologics
- Nuo Therapeutics
- Octapharma
- OiDE BetaRevive
- Ology Bioservices
- Omni Lifesciences
- Oncobiomed
- Opexa Therapeutics
- Orchard Therapeutics
- OrganaBio
- Ori Biotech
- Pall Corporation
- PAN-Biotech
- PBS Biotech
- PDC*line Pharma
- PELOBiotech
- PeproTech
- PersonGen BioTherapeutics
- PharmaBio
- Pharmicell
- PhoenixSongs Biologicals
- Pinze Lifetechnology
- Pique Therapeutics
- Pluristem Therapeutics
- Plus Therapeutics
- Poseida Therapeutics
- Precision BioSciences
- Preferred Cell Systems™
- Pregene ShenZhen Biotechnology
- Primorigen Biosciences (Acquired by Nucleus Biologics)
- ProMab Biotechnologies
- Promethera Biosciences
- PromoCell
- Propagenix
- Provia Laboratories
- R&D Systems
- ReachBio Research Labs
- Regeneris Medical
- Regeneus
- ReNeuron
- REPROCELL
- Rexgenero
- RHEACELL
- Riyadh Pharma
- ROKIT Healthcare
- RoosterBio
- Roslin Cell Therapies
- Rousselot Biomedical
- Rubius Therapeutics
- S.Biomedics
- Sangamo Therapeutics
- Sanvitra
- Saronic Biotechnology
- Sartorius
- Sartorius Korea Biotech (A subsidiary of Sartorius)
- Sartorius Stedim Biotech
- ScienCell Research Laboratories
- Scinogy
- Sclnow Biotechnology
- SCM Lifescience
- Sentien Biotechnologies
- Shanghai Bioray Laboratory
- Shanghai Houchao Biotechnology
- Shanghai iCELL Biotechnology
- Shanghai Longyao Biotechnology
- Shanghai Unicar-Therapy Bio-medicine Technology
- Shenzhen BinDeBio
- Shenzhen Hornetcorn Biotechnology
- Sigma-Aldrich
- Sinobioway Cell Therapy
- SMT Bio
- Sorrento Therapeutics
- SQZ Biotechnologies
- Stem Cell Arabia
- STEMCELL Technologies
- StemImmune
- Stemmatters
- Super-T Cell Cancer Company
- Surface Oncology
- Tactiva Therapeutics
- Taiwan Bio Therapeutics
- Takara Bio
- TapImmune
- Targazyme
- TC BioPharm
- TCR2 Therapeutics
- Tella
- Tessa Therapeutics
- The Discovery Labs Center for Breakthrough Medicines
- Thermo Fisher Scientific
- ThermoGenesis
- Tianhe Stem Cell Biotechnologies
- Tianjin Ever Union Biotechnology
- TiGenix
- TILT Biotherapeutics
- Tmunity Therapeutics
- TNK Therapeutics
- TotipotentRX
- TRACT Therapeutics
- TransCure BioServices
- TransGen Biotech
- TreeFrog Therapeutics
- TRINOVA BIOCHEM
- Triumvira Immunologics
- TVAX Biomedical
- U.S. Stem Cell
- United States Biological
- Universal Cells
- Vaccinogen
- Vericel
- VetStem Biopharma
- Vinasets
- ViroMed
- Viscofan BioEngineering
- Vitro Biopharma
- Vivo Bio Tech
- Waisman Biomanufacturing
- West Biotherapy
- WiCell
- Wissenschaftlicher Service Pharma (WiSP)
- Worchester Polytechnic Institute
- Wuhan Sian Medical Technology
- Xcell Therapeutics
- Xcelthera
- XEME Biopharma
- ZellBio
- Zelluna Immunotherapy
- Ziopharm Oncology
Methodology

LOADING...
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 250 |
| Published | December 2025 |
| Forecast Period | 2025 - 2035 |
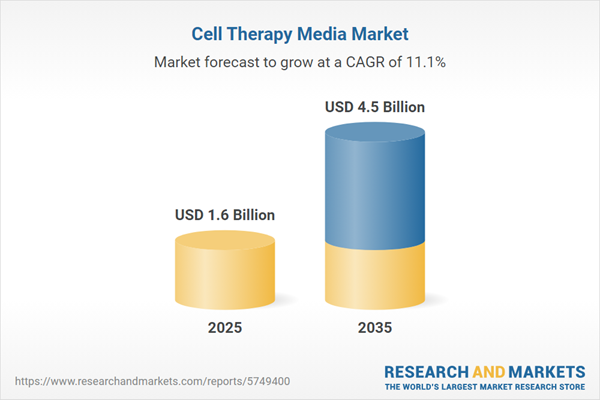
| Estimated Market Value ( USD | $ 1.6 Billion |
| Forecasted Market Value ( USD | $ 4.5 Billion |
| Compound Annual Growth Rate | 11.1% |
| Regions Covered | Global |