Contract development & manufacturing organisations (CDMOs) provide comprehensive services to pharmaceutical and biotechnology companies, including drug development, manufacturing, and packaging. By partnering with CDMOs, pharmaceutical and biotech companies can streamline their operations, reduce costs, and focus on their core competencies. CDMOs are equipped with the necessary infrastructure, expertise, and regulatory compliance to develop and manufacture a wide range of pharmaceutical products, from active pharmaceutical ingredients (APIs) to finished dosage forms. The contract development & manufacturing organisation (CDMO) market 's growth can be attributed to several factors, including the increasing demand for pharmaceuticals due to the rising prevalence of chronic diseases and an expanding geriatric population. This demand has prompted pharmaceutical and biotech companies to seek cost-effective and efficient solutions to develop and manufacture their products, driving the growth of the contract development & manufacturing organisation (CDMO) market.
Another key driver for the contract development & manufacturing organisation (CDMO) market is the growing focus on research and development (R&D) in the pharmaceutical and biotechnology sectors. As companies strive to discover and develop innovative drugs and therapies, they increasingly rely on the expertise and capabilities of CDMOs to accelerate the drug development process and reduce time-to-market. This trend has led to a surge in demand for CDMO services, particularly for early-stage development and clinical trial materials manufacturing.
The increasing adoption of advanced technologies and the growing trend of outsourcing complex and specialised manufacturing processes are also contributing to the growth of the contract development & manufacturing organisation (CDMO) market. CDMOs have adopted cutting-edge technologies, such as continuous manufacturing, single-use systems, and cell and gene therapy platforms, to offer differentiated and specialised services to their clients. This has not only helped CDMOs stay competitive but also enabled pharmaceutical and biotech companies to access state-of-the-art capabilities without incurring significant capital investments. Such advantages and applications are expected to propel the growth of the contract development & manufacturing organisation (CDMO) market.
Market Segmentation
The market can be divided on the basis of service offered, CMO, CRO, and region.Market Bifurcation by Service Offered:
- CMO
- CRO
Market Division by CMO:
- API Manufacturing
- Finished Product Manufacturing
Breakup by Type
- Solid
- Liquid
- Injectable
- Packaging
- Others
Market Segregation by CRO:
- Discovery
- Preclinical
Clinical
Breakup by Type
- Phase I
- Phase II
- Phase III
- Phase IV
Laboratory Services
Breakup by Type
- Bioanalytical Services
- Analytical Services
- Others
Market Breakup by Region:
- North America
- Europe
- Asia Pacific
- Latin America
- Middle East and Africa
Competitive Landscape
The report looks into the market shares, plant turnarounds, capacities, investments, and acquisitions and mergers, among other major developments, of the global contract development & manufacturing organisation companies. Some of the major key players explored in the report are as follows:- Lonza Group Ltd
- Catalent, Inc
- Thermo Fisher Scientific, Inc
- Jubilant Pharmova Ltd
- Aenova Holding GmbH
- Boehringer Ingelheim International GmbH
- Pfizer Inc
- CordenPharma GmbH
- Recipharm AB
- Siegfried Holding AG
- Curia
- Fabbrica Italiana Sintetici S.pA
- Fareva SA
Table of Contents
Companies Mentioned
- Lonza Group Ltd
- Catalent, Inc.
- Thermo Fisher Scientific, Inc.
- Jubilant Pharmova Ltd
- Aenova Holding GmbH
- Boehringer Ingelheim International GmbH
- Pfizer Inc.
- CordenPharma GmbH
- Recipharm AB
- Siegfried Holding AG
- Curia
- Fabbrica Italiana Sintetici S.p.A
- Fareva SA
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 141 |
| Published | March 2023 |
| Forecast Period | 2023 - 2028 |
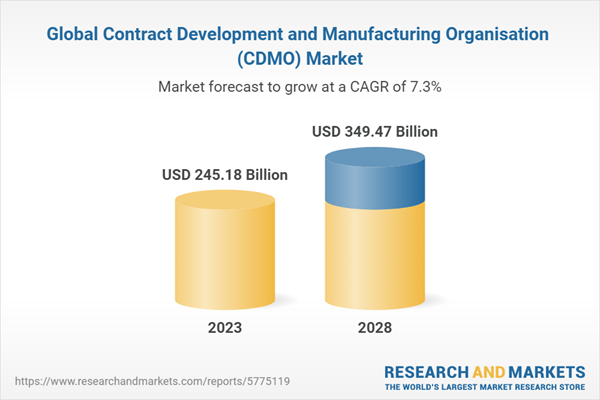
| Estimated Market Value ( USD | $ 245.18 Billion |
| Forecasted Market Value ( USD | $ 349.47 Billion |
| Compound Annual Growth Rate | 7.3% |
| Regions Covered | Global |
| No. of Companies Mentioned | 13 |