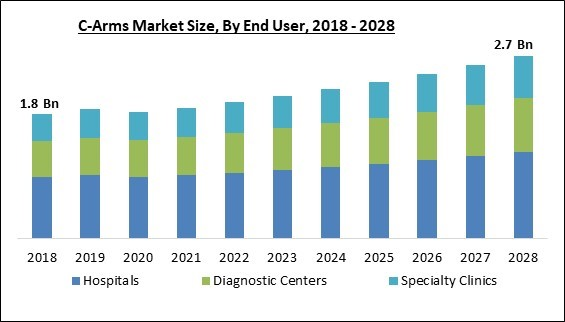
The Global C-Arms Market size is expected to reach $2.7 billion by 2028, rising at a market growth of 5.0% CAGR during the forecast period.
The C-Arm is a flexible medical imaging tool that may be utilized in various clinic operating rooms. The development of the device has resulted from X-ray technology, and thename of the device comes from the C-shaped arm that joins the X-ray detector and X-ray source. A flat panel detector image intensifier and a generator designated X-ray source are both parts of C-Arms. It is frequently used for intra-operative imaging in surgery, orthopedics, vascular surgery,cardiology, and traumatology.
The tool gives the surgeon real-time, high-resolution X-ray images to check on the patient's condition at any stage of the procedure. A C-Arm device is a scanner intensifier for imaging. When performing surgical, orthopedic, and emergency care operations, C-Arms, which have radiographic capabilities, are generally employed for intraoperative imaging. When used for fluoroscopy, the C-Arm is also frequently referred to as a portable x-ray.
Fluoroscopy techniques are medical practices that enable real-time image capture inside certain body sections. X-rays are released by the generator and enter the patient's body. The image detector or intensifier transforms the x-rays into a visual image on the C-Arm monitor. The c-shaped connectionallows for movement in all directions, including horizontally, vertically, as well as around the swivel axis, enabling practically any angle to create an x-ray image of the patient.
Real-time imaging provided by C-Arms enables medical professionals to see a patient's inside organs while performing surgery. As a result, they may be able to identify illnesses more precisely and carry out procedures more securely. The portability of C-Arms makes it simple to transfer them to the operating room.
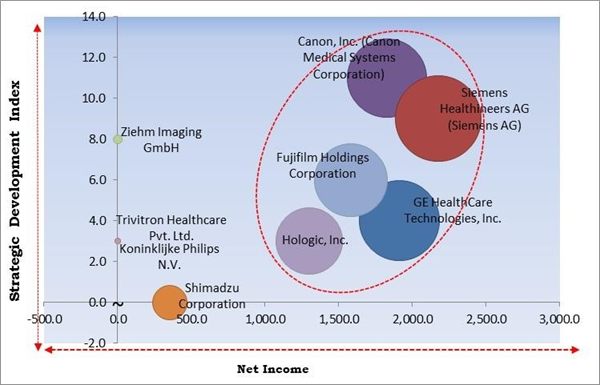
The major strategies followed by the market participants are Product Launches. Based on the Analysis presented in the Cardinal matrix; Canon, Inc. (Canon Medical Systems Corporation), Siemens Healthineers AG (Siemens AG), Hologic, Inc., Fujifilm Holdings Corporation, and GE HealthCare Technologies, Inc. are the forerunners in the C-Arms Market. Companies such as Trivitron Healthcare Pvt. Ltd. and Ziehm Imaging GmbH are some of the key innovators in C-Arms Market.
The market research report covers the analysis of key stake holders of the market. Key companies profiled in the report include Canon, Inc. (Canon Medical Systems Corporation), Siemens Healthineers AG (Siemens AG), Koninklijke Philips N.V., GE HealthCare Technologies, Inc., Fujifilm Holdings Corporation, Shimadzu Corporation, Hologic, Inc., Ziehm Imaging GmbH, Trivitron Healthcare Pvt. Ltd., and Nanjing Prelove Medical Equipment Co., Ltd.
The C-Arm is a flexible medical imaging tool that may be utilized in various clinic operating rooms. The development of the device has resulted from X-ray technology, and thename of the device comes from the C-shaped arm that joins the X-ray detector and X-ray source. A flat panel detector image intensifier and a generator designated X-ray source are both parts of C-Arms. It is frequently used for intra-operative imaging in surgery, orthopedics, vascular surgery,cardiology, and traumatology.
The tool gives the surgeon real-time, high-resolution X-ray images to check on the patient's condition at any stage of the procedure. A C-Arm device is a scanner intensifier for imaging. When performing surgical, orthopedic, and emergency care operations, C-Arms, which have radiographic capabilities, are generally employed for intraoperative imaging. When used for fluoroscopy, the C-Arm is also frequently referred to as a portable x-ray.
Fluoroscopy techniques are medical practices that enable real-time image capture inside certain body sections. X-rays are released by the generator and enter the patient's body. The image detector or intensifier transforms the x-rays into a visual image on the C-Arm monitor. The c-shaped connectionallows for movement in all directions, including horizontally, vertically, as well as around the swivel axis, enabling practically any angle to create an x-ray image of the patient.
Real-time imaging provided by C-Arms enables medical professionals to see a patient's inside organs while performing surgery. As a result, they may be able to identify illnesses more precisely and carry out procedures more securely. The portability of C-Arms makes it simple to transfer them to the operating room.
COVID-19 Impact Analysis
Significant delays weremade in non-urgent medical procedures due to major interruptions in their respective supply chains and industrial processes brought on by several precautionary lockdowns. Demand and supply in the market were downgraded by supply chain problems. The situation became even worse as a result of significant disruptions to their individual industrial and supply-chain operations brought on by numerous precautionary lockdowns. However, elective surgery waiting times decreased by as much as one-third, and the elective surgery rate increased by one-fifth following the lockdown, offering opportunities for growth. Therefore, the COVID-19 pandemic had a negative impact on the C-Arms market.Market Growth Factors
Rising prevalence of acute and chronic diseases and disorders, and injuries
Seventeen million people worldwide succumb to an NCD before they turn 70 each year. Out of all NCD deaths, 77% occur in low- and middle-income nations, accounting for 86% of these deaths. Chronic respiratory diseases (4.1 million), cancers (9.3 million), and diabetes account for most NCD deaths or 17.9 million people annually. Also, the increased number of traffic incidents and other orthopedic injuries expedites the requirement for C-Arms. Therefore, as the prevalence of these diseases and injuries rises, it will propel the demand for efficient and fast diagnostic devices. This, in turn, drives the growth of the C-Arms market.Growing research and development and technological advancement
Certain other developments like vascular 3D image guiding, movable mini C-Arm, 3-D surgical imaging system, and multidisciplinary functionality are all possible due to the increased R&D. Furthermore, the increasing FDA approval rates of these developments have further prompted manufacturers to innovate. For example, the FDA has approved the Cios Flow, a device having multidisciplinary versatility, which ensures excellent system usage and capacity. Furthermore, its features allow for the simpler, more effective operation to enhance patient care in the OR. Hence, the rising R&D efforts by the market participants promote the market's growth.Market Restraining Factors
Low return on investment and high cost of R&D
High expenditures associated with R&D capabilities, constrained infrastructure facilities, and expensive maintenance andinstallation costs are anticipated to hinder the demand for fixed and mobile C-Arms. Also, the market is anticipated to face challenges from the penetration of technology in developing nations as well as a lack of suitable reimbursement scenarios. Furthermore, the development of fixed and mobile C-Arms is furtherhampered by technological problems, a high-risk aspect related to emergency cases, a lack of qualified doctors in developing and undeveloped areas, and an inadequate infrastructure in many nations. Therefore, the expansion of the C-Arms market is anticipated to be threatened by these factorsthroughout the forecast period.Type Outlook
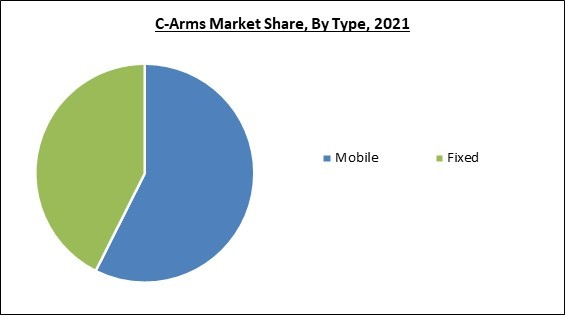
Based on type, the C-Arms market is categorized into mobile and fixed. The fixed segment procured a considerable growth rate in the C-Arms market in 2021. The segment's growth is aided by the Centers for Medicare and Medicaid Services (CMS) approval of specific cardiovascular procedures. In addition, fixed C-Arm uses have changed due to ongoing technological and clinical developments. In addition to cardiology andradiology departments, they can be put in surgery centers with access to various medical specialties, where conventional film X-rays were formerly employed to deliver imaging for surgeonsApplication Outlook
On the basis of application, the C-Arms market is divided into cardiology, gastroenterology, neurology, orthopedic & trauma, oncology, and others. The neurology segment witnessed a promising growth rate in the C-Arms market in 2021. For directing minimally invasive procedures and tracking results, the C-Arm system's data is crucial. For example, in neurosurgery, accuracy is crucial since even the smallest modification can have a big impact on the clinical andfunctional outcomes of the patient. As a result, precise imaging of the brain anatomy, which is necessary for treatments, can be provided using high-resolution C-Arm technology.End-user Outlook
Based on end user, the C-Arms market is segmented into hospitals, diagnostic centers, and specialty clinics. The hospitals segment garnered the maximum revenue share in the C-Arms market in 2021. Hospitals are the principal end users of C-Arms systems and the primary customers of C-Arms device makers, which accounts for the segment's growth. Mobile imaging is required for several medical procedures, such as orthopedic, vascular, and gastrointestinal surgeries, which drives the need for C-Arms equipment in hospitals. As the frequency of interventional therapies rises, so does the demand for medical imaging.Regional Outlook
On the basis of region, the C-Arms market is analyzed across North America, Europe, Asia Pacific, and LAMEA. The North America segment acquired the highest revenue share in the C-Arms market in 2021. The emergence of several significant important players and the rapid advancement of technology in theregion fuel market expansion. In addition, the increased demand for minimally invasive surgical treatments, the quick expansion of the elderly population, the introduction of technologically advanced C-Arms devices, and improved healthcare infrastructure are all important factors propelling the regionalC-Arms market.The Cardinal Matrix - C-Arms Market Competition Analysis
The major strategies followed by the market participants are Product Launches. Based on the Analysis presented in the Cardinal matrix; Canon, Inc. (Canon Medical Systems Corporation), Siemens Healthineers AG (Siemens AG), Hologic, Inc., Fujifilm Holdings Corporation, and GE HealthCare Technologies, Inc. are the forerunners in the C-Arms Market. Companies such as Trivitron Healthcare Pvt. Ltd. and Ziehm Imaging GmbH are some of the key innovators in C-Arms Market.
The market research report covers the analysis of key stake holders of the market. Key companies profiled in the report include Canon, Inc. (Canon Medical Systems Corporation), Siemens Healthineers AG (Siemens AG), Koninklijke Philips N.V., GE HealthCare Technologies, Inc., Fujifilm Holdings Corporation, Shimadzu Corporation, Hologic, Inc., Ziehm Imaging GmbH, Trivitron Healthcare Pvt. Ltd., and Nanjing Prelove Medical Equipment Co., Ltd.
Strategies Deployed in C-Arms Market
Partnerships, Collaborations and Agreements:
- Mar-2023: Ziehm Imaging, Inc. came into partnership with NXC Imaging, a provider of integrated diagnostic solutions. This partnership would enable NXC Imaging to provide their customers with quality C-arm imaging by distributing the comprehensive suite of advanced C-arms consisting of the compact Ziehm Solo to the game-changing intraoperative Ziehm Vision RFD 3D.
- May-2022: Siemens Healthineers partnered with PrecisionOS, a provider of virtual reality surgical training for the medical industry. Under this partnership, PrecisionOS would provide virtual reality (VR) training to help technicians and surgeons to practice the usage of Siemens Healthineers' mobile 3D C-arm Cios Spin for surgical workflow guidance and intraoperative quality control.
- Apr-2022: GE Healthcare joined hands with Medtronic, a company engaged in offering medical technology, services, and solutions. Under this collaboration, both companies would focus on the specific requirements and demand for care at Office-Based Labs and Ambulatory Surgery Centers. Moreover, customers would be able to access an extensive product suite, financial solutions, and exceptional service.
- Apr-2021: Ziehm Imaging partnered with Carestream Health, a global provider of medical imaging systems. Carestream would add the Ziehm Vision RFD C-arm, a mobile C-arm into its growing innovative product portfolio. The product would increase Carestream’s mobile and fluoroscopic product portfolio to benefit more healthcare providers.
- Apr-2021: Siemens Healthineers partnered with Radlink, Inc., a manufacturer of medical devices. Through this partnership, both companies would design a completely integrated app-based solution to provide orthopedic surgical software through the Cios Mobile C-arm.
Product Launches and Product Expansions:
- Mar-2023: Canon Medical introduced the Celex, the latest versatile, multi-purpose X-ray system. The product delivers a broad range of advanced DR imaging abilities. Also, the product integrates modern imaging with unique positioning flexibility and shows an actual hybrid that provides optimum capacity utilization.
- Nov-2021: FUJIFILM Healthcare Americas launched Persona CS mobile fluoroscopy system, the compact mobile C-arm imaging solution developed for quick and smooth positioning in operating room environments. Through this launch, the company would provide customers with two powerful C-arm solutions that serve large hospitals and ambulatory surgery centers, and pain clinics.
Mergers and Acquisitions:
- Jul-2022: Canon Medical USA took over NXC Imaging, a distributor and service provider of capital medical equipment, consisting of C-Arms, CT, Vascular, Ultrasound, and X-Ray. The acquisition would strengthen Canon Medical Systems USA's sales and service channels in the US Upper Midwest of its imaging products.
- Mar-2022: Canon Medical Systems Corporation completed the acquisition of Nordisk Røntgen Teknik A/S, a manufacturer of medical equipment. This acquisition would allow Canon Medical to strengthen its offering both in multi-purpose fluoroscopy and interventional gastro. Also, with this acquisition Canon Medical would have access to European-based technology and manufacturing and the chance to deploy advanced technology for multipurpose fluoroscopy.
Approvals & Trials:
- Mar-2021: GE Healthcare received FDA approval for OEC 3D, a surgical imaging system capable of 3D and 2D imaging. The product would serve clinicians who require better 3D volumetric images rapidly at the time of intraoperative procedures. Additionally, OEC 3D is created in the OEC Elite C-arm platform, the known performance and functionality of the OEC 3D C-arm would make 3D imaging routine for joint replacement and complex spine procedures.
Scope of the Study
By End-user
- Hospitals
- Diagnostic Centers
- Specialty Clinics
By Type
- Mobile
- Fixed
By Application
- Orthopedic & Trauma
- Oncology
- Cardiology
- Gastroenterology
- Neurology
- Others
By Geography
- North America
- US
- Canada
- Mexico
- Rest of North America
- Europe
- Germany
- UK
- France
- Russia
- Spain
- Italy
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- Singapore
- Malaysia
- Rest of Asia Pacific
- LAMEA
- Brazil
- Argentina
- UAE
- Saudi Arabia
- South Africa
- Nigeria
- Rest of LAMEA
Key Market Players
List of Companies Profiled in the Report:
- Canon, Inc. (Canon Medical Systems Corporation)
- Siemens Healthineers AG (Siemens AG)
- Koninklijke Philips N.V.
- GE HealthCare Technologies, Inc.
- Fujifilm Holdings Corporation
- Shimadzu Corporation
- Hologic, Inc.
- Ziehm Imaging GmbH
- Trivitron Healthcare Pvt. Ltd.
- Nanjing Prelove Medical Equipment Co., Ltd.
Unique Offerings
- Exhaustive coverage
- The highest number of Market tables and figures
- Subscription-based model available
- Guaranteed best price
- Assured post sales research support with 10% customization free
Table of Contents
Chapter 1. Market Scope & Methodology
Chapter 2. Market Overview
Chapter 3. Competition Analysis - Global
Chapter 4. Global C-Arms Market by End-user
Chapter 5. Global C-Arms Market by Type
Chapter 6. Global C-Arms Market by Application
Chapter 7. Global C-Arms Market by Region
Chapter 8. Company Profiles
Companies Mentioned
- Canon, Inc. (Canon Medical Systems Corporation)
- Siemens Healthineers AG (Siemens AG)
- Koninklijke Philips N.V.
- GE HealthCare Technologies, Inc.
- Fujifilm Holdings Corporation
- Shimadzu Corporation
- Hologic, Inc.
- Ziehm Imaging GmbH
- Trivitron Healthcare Pvt. Ltd.
- Nanjing Prelove Medical Equipment Co., Ltd.