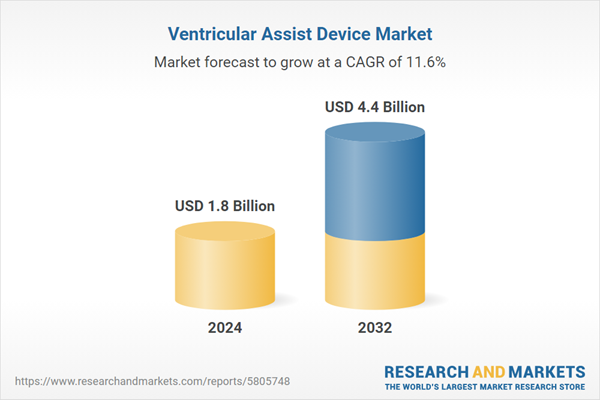
Global Ventricular Assist Device Market- Analysis
A ventricular assist device is a mechanical pump used to support heart function and blood flow in individuals with weakened hearts. These devices are particularly crucial for patients awaiting heart transplants or those who are ineligible for transplantation. The VAD market is expanding due to technological advancements, growing awareness of heart failure treatments, and the increasing availability of these devices in healthcare settings worldwide.Market Drivers
Rising Prevalence of Heart Failure: The increasing incidence of heart failure globally is a primary driver of the ventricular assist devices market. With the growing number of patients suffering from cardiovascular diseases, the demand for advanced treatment options like ventricular assist devices is on the rise, propelling market growth.Advancements in VAD Technology: Continuous advancements in ventricular assist device technology, including the development of smaller, more efficient, and durable devices, are significantly driving the market. These innovations enhance patient outcomes and reduce the complications associated with older devices.
Growing Geriatric Population: The global ageing population is contributing to the rising demand for ventricular assist devices. As the elderly population is more prone to heart conditions, the need for effective heart support devices is increasing, thereby driving market expansion.
Increasing Number of Heart Transplants: The rising number of heart transplants worldwide is bolstering the demand for ventricular assist devices as bridge-to-transplant devices. These devices play a critical role in keeping patients stable while they await a donor's heart.
Market Challenges
Risk of Complications: Despite technological advancements, ventricular assist devices carry risks of complications such as infection, blood clots, and device malfunction. These potential risks can deter patients and healthcare providers from opting for ventricular assist devices, affecting market growth.Limited Awareness in Emerging Markets: There is limited awareness and availability of ventricular assist devices in emerging markets, which hampers market growth. The lack of infrastructure and trained healthcare professionals to manage ventricular assist devices further exacerbates this challenge.
Stringent Regulatory Requirements: Navigating the complex regulatory landscape for medical devices, including ventricular assist devices, is a significant challenge for manufacturers. The stringent approval processes and varying regulations across different regions can delay product launches and market entry.
Future Opportunities
Development of Next-Generation VADs: The development of next-generation ventricular assist devices that are smaller, more efficient, and offer improved patient outcomes presents significant growth opportunities. Innovations in battery life, wireless technology, and biocompatibility are expected to drive market expansion.Collaborations and Partnerships: Collaborations between medical device manufacturers, research institutions, and healthcare providers can lead to the development of innovative ventricular assist device solutions. These partnerships can also help in expanding market reach and improving patient access to advanced treatments.
Expansion of Ventricular Assist Devices Applications: The expansion of ventricular assist device applications beyond heart failure, including in cases of myocardial infarction and other severe cardiovascular conditions, presents growth opportunities. Broader applications can attract a wider patient population and drive market growth.
Global Ventricular Assist Device Market Trends
Shift Towards Minimally Invasive Procedures: The trend towards minimally invasive ventricular assist device implantation is gaining momentum, driven by the benefits of reduced recovery times, lower complication rates, and improved patient outcomes. These procedures are increasingly preferred in clinical practice, as they offer less trauma and quicker recovery, making ventricular assist devices a more viable option for a broader range of patients with heart failure.Integration of Artificial Intelligence (AI): The integration of artificial intelligence (AI) and machine learning into the management and monitoring of ventricular assist devices is a significant emerging trend. AI enhances ventricular assist devices' performance by enabling real-time data analytics, predictive maintenance, and personalised treatment plans, thereby improving patient safety and device reliability. This trend is poised to revolutionise how VADs are monitored and managed in clinical settings.
Rising Demand for Durable Mechanical Circulatory Support: With the increasing life expectancy of patients living with heart failure, there is a growing demand for durable mechanical circulatory support devices like ventricular assist devices. This trend is driving the development of long-lasting devices capable of providing extended support, ensuring that patients with chronic heart conditions can maintain a better quality of life over longer periods.
Focus on Portable and Wearable VADs: The market is seeing a surge in demand for portable and wearable ventricular assist devices that offer patients greater mobility and independence. These devices are designed to be more user-friendly and less intrusive, aligning with the broader trend towards enhancing patient quality of life. The development of these compact devices reflects the growing emphasis on patient-centred care in the treatment of heart failure.
Advances in Biocompatible Materials: Advancements in biocompatible materials used in ventricular assist devices are significantly improving the safety and efficacy of these devices. The development of materials that minimise the risk of blood clots, infections, and other complications is a key trend, contributing to better patient outcomes and extending the longevity of ventricular assist devices. These innovations are crucial for enhancing the overall success of ventricular assist device therapy.
Global Ventricular Assist Device Market Segmentation
Market Breakup by Product Type
- Left Ventricular Assist Device (LVAD)
- Right Ventricular Assist Device (RVAD)
- Biventricular Assist Device (BIVAD)
Market Breakup by Type of Flow
- Pulsatile Flow
- Continuous Flow
Market Breakup by Application
- Bridge to Transplant (BTT)
- Bridge to Candidacy (BTC)
Market Breakup by End User
- Hospitals
- Ambulatory Surgical Centre
- Specialty Clinics
- Others
Market Breakup by Region
- North America
- Europe
- Asia Pacific
- Latin America
- Middle East and Africa
Global Ventricular Assist Device Market Competitive Landscape
The global ventricular assist device market features key players such as Abiomed Inc., BiVACOR Inc., Abbott Laboratories, Medtronic plc, Terumo Corporation, Fisher & Paykel Healthcare Limited, Evaheart, Inc., Calon Cardio-Technology Ltd, Berlin Heart GmbH, AdjuCor GmbH, Jarvik Heart Inc., SynCardia Systems LLC, and CALON CARDIO-TECHNOLOGY LTD. These companies are at the forefront of innovation, focusing on developing advanced VAD technologies, expanding their product portfolios, and enhancing patient outcomes. Through continuous research and development, these market leaders are driving the evolution of cardiovascular treatment, ensuring the availability of life-saving devices globally.Key Questions Answered in the Report
- What factors driving the adoption of ventricular assist devices in the global healthcare market?
- How are technological advancements influencing the development and performance of next-generation ventricular assist devices?
- What role does the growing geriatric population play in the increased demand for ventricular assist devices?
- How do the risks associated with ventricular assist device implantation, such as infection and device malfunction, impact market growth?
- In what ways are regulatory challenges affecting the global expansion of the ventricular assist devices market?
- How is the rising prevalence of heart failure globally contributing to the demand for ventricular assist devices?
- What emerging trends are shaping the future of ventricular assist devices technology, particularly in terms of AI integration and biocompatible materials?
- How do portable and wearable ventricular assist devices enhance patient mobility and quality of life, and what is their impact on market dynamics?
- What are the primary differences in market growth and adoption between continuous flow and pulsatile flow ventricular assist devices?
- How is the expansion of ventricular assist device applications beyond heart failure, such as in myocardial infarction, influencing the market landscape?
- What strategies are key players in the ventricular assist devices market employing to expand their product portfolios and enhance patient outcomes?
Key Benefits for Stakeholders
The industry report offers a comprehensive quantitative analysis of various market segments, historical and current market trends, market forecasts, and dynamics of the global ventricular assist devices market from 2017-2032.- The research report provides the latest information on the market drivers, challenges, and opportunities in the global ventricular assist devices market.
- The study maps the leading, as well as the fastest-growing, regional markets, enabling stakeholders to identify key country-level markets within each region.
- Porter's five forces analysis assists stakeholders in assessing the impact of new entrants, competitive rivalry, supplier power, buyer power, and the threat of substitution. It helps stakeholders analyse the level of competition within the global ventricular assist devices industry and its attractiveness.
- The competitive landscape section allows stakeholders to understand their competitive environment and provides insight into the current positions of key players in the market.
This product will be delivered within 5-7 business days.
Table of Contents
Companies Mentioned
- Abiomed Inc.
- BiVACOR Inc.
- Abbott Laboratories
- Medtronic plc
- Terumo Corporation
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 200 |
| Published | October 2024 |
| Forecast Period | 2024 - 2032 |
| Estimated Market Value ( USD | $ 1.8 Billion |
| Forecasted Market Value ( USD | $ 4.4 Billion |
| Compound Annual Growth Rate | 11.6% |
| Regions Covered | Global |
| No. of Companies Mentioned | 5 |