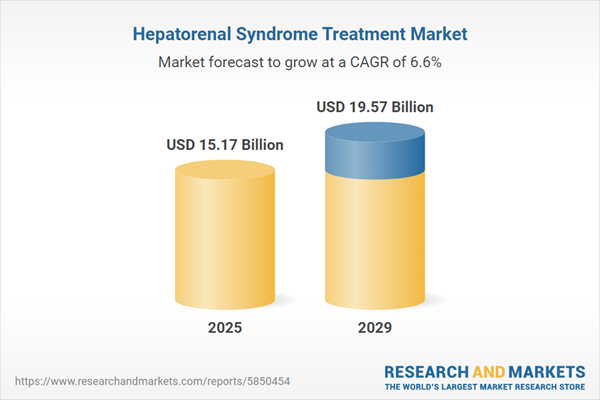
The hepatorenal syndrome treatment market size is expected to see strong growth in the next few years. It will grow to $19.57 billion in 2029 at a compound annual growth rate (CAGR) of 6.6%. The growth in the forecast period can be attributed to rise in renewable energy projects, growing demand for high-performance alloys, focus on sustainable manufacturing, expansion of oil and gas exploration, demand for high-performance components. Major trends in the forecast period include integration of industry 4.0 technologies, research and development in forging techniques, customization for specific applications, automation in forging processes, innovations in heating and cooling technologies.
The forecast of 6.6% growth over the next five years reflects a modest reduction of 0.2% from the previous estimate for this market. This reduction is primarily due to the impact of tariffs between the US and other countries. Tariff escalations could challenge U.S. critical care hepatology by driving up the cost of vasoconstrictor therapies and renal replacement filters sourced from Sweden and Brazil, raising mortality risks in cirrhotic patients and increasing intensive care unit treatment costs. The effect will also be felt more widely due to reciprocal tariffs and the negative effect on the global economy and trade due to increased trade tensions and restrictions.
The rising incidence of patients with liver damage or cirrhosis is expected to drive the growth of the hepatorenal syndrome treatment market in the coming years. Cirrhosis is a serious liver disease characterized by the permanent replacement of healthy liver tissue with scar tissue. Treatment for hepatorenal syndrome focuses on preserving kidney function while alleviating the symptoms and negative effects of the condition. These strategies can enhance overall health and improve eligibility for liver transplant surgery. For example, a report published in July 2023 by the British Liver Trust, a UK-based charity organization, revealed that hospital admissions for liver disease in England increased by 22% in just one year, rising from 67,458 in 2021 to 82,290 in the financial year ending in 2022. Consequently, the increase in cases of liver damage or cirrhosis is driving the growth of the hepatorenal syndrome treatment market.
The increasing number of fast-track and novel drug designations is expected to drive the growth of the hepatorenal syndrome treatment market in the future. Fast-track and novel drug designations are regulatory pathways designed to accelerate the development, review, and approval of drugs for serious conditions with unmet medical needs, facilitating quicker access to innovative therapies for patients. The rise in these designations aims to expedite the development of treatments for hepatorenal syndrome by addressing the urgent need for effective therapies, potentially enhancing patient outcomes and fulfilling unmet medical needs associated with this condition. For example, a report from the Food and Drug Administration (FDA), a U.S. regulatory agency, stated that in 2022, 37 new drugs, known as novel drugs, were approved, which had never before been approved or marketed in the U.S. Among these, the Center for Drug Evaluation and Research (CDER) granted approvals for 28 (76%) during their first review cycle. Thus, the rise in fast-track and novel drug designations is propelling the growth of the hepatorenal syndrome treatment market.
An increase in healthcare spending is projected to drive the growth of the hepatorenal syndrome treatment market in the coming years. Healthcare spending encompasses the total expenditures on healthcare across all sectors of an economy, including hospitals, home health services, prescription drugs, nursing homes, and individual healthcare. As healthcare spending increases, more resources may be allocated to the research and development of innovative and more effective treatments for hepatorenal syndrome (HRS). Additionally, higher spending could lead to improved availability of tools for HRS screening and early detection, which may facilitate earlier interventions and better patient outcomes. For instance, a report from the Office for National Statistics, a UK-based government department, indicated that total healthcare expenditure increased by 5.6% in nominal terms from 2022 to 2023, compared to a growth rate of just 0.9% in 2022. Therefore, the rise in healthcare spending is a significant factor driving the growth of the hepatorenal syndrome treatment market.
A prominent trend in the hepatorenal syndrome treatment market is the increasing focus on research and development activities. Companies operating in this market are intensifying their efforts in research and development to maintain their market positions. For example, Mallinckrodt Pharmaceuticals, in September 2022, received FDA approval for Terlivaz, an injection to help adults with hepatorenal syndrome's kidney function, supported by the Phase 3 CONFIRM trial. This emphasis on research and development is vital for advancing treatment options for hepatorenal syndrome, resulting in improved patient outcomes.
Major companies in the hepatorenal syndrome treatment market are strategically focusing on advanced products such as hepatorenal drug solutions to enhance patient outcomes and address unmet medical needs. Hepatorenal drug solutions refer to innovative pharmaceuticals or treatments specifically designed to target and address the intricate pathology of hepatorenal syndrome. For instance, Ocelot Bio Inc., in August 2022, received orphan drug designation for its lead candidate OCE-205, addressing the urgent unmet medical needs of hepatorenal syndrome patients through a distinct mechanism of action. This strategic focus on advanced solutions contributes to improved efficacy and better management of hepatorenal syndrome, offering hope to patients with this complex condition affecting the liver and kidneys.
Major companies operating in the hepatorenal syndrome treatment market include BioVie Inc., Cumberland Pharmaceuticals Inc., Mallinckrodt Pharmaceuticals, Orphan Therapeutics LLC, Baxter International Inc., La Jolla Pharmaceutical Company, Noorik Biopharmaceuticals AG, Novartis AG, Zydus Lifesciences Limited, Ocelot Bio Inc., New Medicon Pharma Lab Private Limited, Arrowhead Pharmaceuticals, Stealth BioTherapeutics Inc., Eli Lilly and Company, Teva Pharmaceutical Industries Ltd., Vertex Pharmaceuticals Inc., Johnson & Johnson, Boehringer Ingelheim GmbH, Catalyst Pharmaceuticals Inc., Bayer AG, Gilead Sciences Inc.
North America was the largest region in the hepatorenal syndrome treatment market in 2024. Asia-Pacific is expected to be the fastest-growing region in the global hepatorenal syndrome treatment market during the forecast period. The regions covered in the hepatorenal syndrome treatment market report are Asia-Pacific, Western Europe, Eastern Europe, North America, South America, Middle East, Africa. The countries covered in the hepatorenal syndrome treatment market report are Australia, Brazil, China, France, Germany, India, Indonesia, Japan, Russia, South Korea, UK, USA, Canada, Italy, Spain.
Note that the outlook for this market is being affected by rapid changes in trade relations and tariffs globally. The report will be updated prior to delivery to reflect the latest status, including revised forecasts and quantified impact analysis. The report’s Recommendations and Conclusions sections will be updated to give strategies for entities dealing with the fast-moving international environment.
The sudden escalation of U.S. tariffs and the resulting trade tensions in spring 2025 are having a significant impact on the pharmaceutical sector. Companies are grappling with higher costs on imported active pharmaceutical ingredients (APIs), glass vials, and laboratory equipment - many of which have limited alternative sources. Generic drug manufacturers, already operating with minimal profit margins, are particularly affected, with some scaling back production of low-margin medications. Biotech firms are also experiencing delays in clinical trials due to shortages of specialized reagents linked to tariffs. In response, the industry is shifting API production to regions like India and Europe, building up inventory reserves, and advocating for tariff exemptions on essential medicines.
The hepatorenal syndrome treatment market research report is one of a series of new reports that provides hepatorenal syndrome treatment optical components market statistics, including hepatorenal syndrome treatment optical components industry global market size, regional shares, competitors with a hepatorenal syndrome treatment optical components market share, detailed hepatorenal syndrome treatment optical components market segments, market trends and opportunities, and any further data you may need to thrive in the hepatorenal syndrome treatment optical components industry. This hepatorenal syndrome treatment optical components market research report delivers a complete perspective of everything you need, with an in-depth analysis of the current and future scenario of the industry.
Hepatorenal syndrome (HRS) is a multiorgan disorder characterized by acute kidney injury in patients with severe liver disease. Individuals with this syndrome exhibit signs and symptoms of liver failure along with reduced urine output, leading to oliguria.
The main types of hepatorenal syndrome treatment include type 1 hepatorenal syndrome and type 2 hepatorenal syndrome. Type 2 hepatorenal syndrome is characterized by a moderate and steady decline in glomerular filtration rate and commonly affects individuals whose liver function is still predominantly intact. Treatment involves the administration of therapeutics and, in some cases, surgical interventions. These treatments are utilized across various end-users, including hospitals and clinics, ambulatory surgical centers, academic and research institutes, among others.
The hepatorenal syndrome treatment market includes revenues earned by providing imaging, blood, and urine tests to evaluate the patient's liver and kidney function. The market value includes the value of related goods sold by the service provider or included within the service offering. Only goods and services traded between entities or sold to end consumers are included.
The market value is defined as the revenues that enterprises gain from the sale of goods and/or services within the specified market and geography through sales, grants, or donations in terms of the currency (in USD, unless otherwise specified).
The revenues for a specified geography are consumption values that are revenues generated by organizations in the specified geography within the market, irrespective of where they are produced. It does not include revenues from resales along the supply chain, either further along the supply chain or as part of other products.
This product will be delivered within 1-3 business days.
Table of Contents
Executive Summary
Hepatorenal Syndrome Treatment Global Market Report 2025 provides strategists, marketers and senior management with the critical information they need to assess the market.This report focuses on hepatorenal syndrome treatment market which is experiencing strong growth. The report gives a guide to the trends which will be shaping the market over the next ten years and beyond.
Reasons to Purchase:
- Gain a truly global perspective with the most comprehensive report available on this market covering 15 geographies.
- Assess the impact of key macro factors such as geopolitical conflicts, trade policies and tariffs, post-pandemic supply chain realignment, inflation and interest rate fluctuations, and evolving regulatory landscapes.
- Create regional and country strategies on the basis of local data and analysis.
- Identify growth segments for investment.
- Outperform competitors using forecast data and the drivers and trends shaping the market.
- Understand customers based on the latest market shares.
- Benchmark performance against key competitors.
- Suitable for supporting your internal and external presentations with reliable high quality data and analysis
- Report will be updated with the latest data and delivered to you along with an Excel data sheet for easy data extraction and analysis.
- All data from the report will also be delivered in an excel dashboard format.
Description
Where is the largest and fastest growing market for hepatorenal syndrome treatment? How does the market relate to the overall economy, demography and other similar markets? What forces will shape the market going forward, including technological disruption, regulatory shifts, and changing consumer preferences? The hepatorenal syndrome treatment market global report answers all these questions and many more.The report covers market characteristics, size and growth, segmentation, regional and country breakdowns, competitive landscape, market shares, trends and strategies for this market. It traces the market’s historic and forecast market growth by geography.
- The market characteristics section of the report defines and explains the market.
- The market size section gives the market size ($b) covering both the historic growth of the market, and forecasting its development.
- The forecasts are made after considering the major factors currently impacting the market. These include: the technological advancements such as AI and automation, Russia-Ukraine war, trade tariffs (government-imposed import/export duties), elevated inflation and interest rates.
- Market segmentations break down the market into sub markets.
- The regional and country breakdowns section gives an analysis of the market in each geography and the size of the market by geography and compares their historic and forecast growth.
- The competitive landscape chapter gives a description of the competitive nature of the market, market shares, and a description of the leading companies. Key financial deals which have shaped the market in recent years are identified.
- The trends and strategies section analyses the shape of the market as it emerges from the crisis and suggests how companies can grow as the market recovers.
Scope
Markets Covered:
1) By Type: Type 1 Hepatorenal Syndrome; Type 2 Hepatorenal Syndrome2) By Treatment: Therapeutics; Surgical Treatment
3) By End-User: Hospitals and Clinics; Ambulatory Surgical Centers; Academic and Research Institutes; Other End-Users
Subsegments:
1) By Type 1 Hepatorenal Syndrome: Intravenous Albumin; Vasopressors; Dialysis; Liver Transplantation2) By Type 2 Hepatorenal Syndrome: Albumin Infusion; Midodrine; Octreotide; Liver Transplantation
Companies Mentioned: BioVie Inc.; Cumberland Pharmaceuticals Inc.; Mallinckrodt Pharmaceuticals; Orphan Therapeutics LLC; Baxter International Inc.; La Jolla Pharmaceutical Company; Noorik Biopharmaceuticals AG; Novartis AG; Zydus Lifesciences Limited; Ocelot Bio Inc.; New Medicon Pharma Lab Private Limited; Arrowhead Pharmaceuticals; Stealth BioTherapeutics Inc.; Eli Lilly and Company; Teva Pharmaceutical Industries Ltd.; Vertex Pharmaceuticals Inc.; Johnson & Johnson; Boehringer Ingelheim GmbH; Catalyst Pharmaceuticals Inc.; Bayer AG; Gilead Sciences Inc.
Countries: Australia; Brazil; China; France; Germany; India; Indonesia; Japan; Russia; South Korea; UK; USA; Canada; Italy; Spain
Regions: Asia-Pacific; Western Europe; Eastern Europe; North America; South America; Middle East; Africa
Time Series: Five years historic and ten years forecast.
Data: Ratios of market size and growth to related markets, GDP proportions, expenditure per capita.
Data Segmentation: Country and regional historic and forecast data, market share of competitors, market segments.
Sourcing and Referencing: Data and analysis throughout the report is sourced using end notes.
Delivery Format: PDF, Word and Excel Data Dashboard.
Companies Mentioned
The companies featured in this Hepatorenal Syndrome Treatment market report include:- BioVie Inc.
- Cumberland Pharmaceuticals Inc.
- Mallinckrodt Pharmaceuticals
- Orphan Therapeutics LLC
- Baxter International Inc.
- La Jolla Pharmaceutical Company
- Noorik Biopharmaceuticals AG
- Novartis AG
- Zydus Lifesciences Limited
- Ocelot Bio Inc.
- New Medicon Pharma Lab Private Limited
- Arrowhead Pharmaceuticals
- Stealth BioTherapeutics Inc.
- Eli Lilly and Company
- Teva Pharmaceutical Industries Ltd.
- Vertex Pharmaceuticals Inc.
- Johnson & Johnson
- Boehringer Ingelheim GmbH
- Catalyst Pharmaceuticals Inc.
- Bayer AG
- Gilead Sciences Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 250 |
| Published | September 2025 |
| Forecast Period | 2025 - 2029 |
| Estimated Market Value ( USD | $ 15.17 Billion |
| Forecasted Market Value ( USD | $ 19.57 Billion |
| Compound Annual Growth Rate | 6.6% |
| Regions Covered | Global |
| No. of Companies Mentioned | 22 |