In Silico Clinical Trials: Introduction
In silico clinical trials are used to perform tests and trials pre-launch of drugs and medicines in the market. The in silico clinical trials, which are also known as virtual clinical trials, are used to perform clinical tests without harming any human or animal. In these clinical trials computer-based models and simulations are used to know about the safety, accuracy, efficiency and outcomes of the treatment before sending them under real trials. With the help of in silico clinical trials, time consumption in comparison to the real trials is less. In in silico clinical trials, the imitation of real medicines takes place, which is further utilized to personalize treatment process and develop personalize treatment plan. It also helps in speeding up the development process of the drug and the time when it would be active in the body.Global In Silico Clinical Trials Market Analysis
The increasing focus on potential of in silico clinical trials in the development process of drug as it provides useful insights before the real human trials, is expected to aid the in-silico clinical trials market growth. This process has the potential of reducing the time, expenditure and effectiveness of the drug development process.Additionally, with the help of in silico clinical trials combined with the patient-specific data, such as medical symptoms from past or genetic data, in order to generate personalized medicine will directly contribute to the market growth. The increasing technological development in the healthcare industry and specifically in in-silico clinical trials has been creating a huge impact on the market as well, propelling the global in-silico clinical trials market expansion. Advanced technologies like artificial intelligence and machine learning are used to perform in-silico clinical trials in order to achieve more accuracy and by processing data which would not be possible to process easily with the use of other traditional methods.
Global In Silico Clinical Trials Market Segmentations
In Silico Clinical Trials Market Report and Forecast 2025-2034” offers a detailed analysis of the market based on the following segments:Market Segmentation by Industry
- Medical Devices
- Pharmaceuticals
Market Breakup by Therapeutic Area
- Oncology
- Infectious Disease
- Hematology
- Cardiology
- Dermatology
- Neurology
- Diabetes
- Others
Market Breakup by Phase
- Phase I
- Phase II
- Phase III
- Phase IV
Market Breakup by Region
- North America
- Europe
- Asia Pacific
- Latin America
- Middle East and Africa
Global In Silico Clinical Trials Market Overview
The major factors driving the market growth include the involvement of advanced technologies such as artificial intelligence and machine learning to achieve more accuracy in the outcomes of these trials. More accuracy will result in the development of more effective drugs and medicines. In-silico clinical trials saves time and are cost-effective as well as they are not being performed on real humans or animals but virtual patients.Additionally, the continuous development to increase the efficiency of software used in the systems for these trials also contributes to the technological advancement in in-silico clinical trials. The market is growing steadily from past few years and is expected to keep growing steadily in future as well. In silico clinical trials helps researchers to simulate the drug responses in virtual patient populations which further helps in the development of personalized medicine approaches and facilitates the optimization of drug regimens for particular patient populations and improving treatment outcomes further contributing to the global in-silico clinical trials market expansion.
Global in Silico Clinical Trials Market: Competitor Landscape
The key features of the market report include patent analysis, grants analysis, clinical trials analysis, funding and investment analysis, partnerships, and collaborations analysis by the leading key players. The major companies in the market are as follows:- Dassault Systèmes SE
- Certara Inc.
- Insilico Medicine
- GNS Healthcare Inc.
- The AnyLogic Company
- Novadiscovery SAS
- InSilicoTrials Technologies SpA
- Immunetrics Inc.
- CATO SMS
- Evotec SE
This product will be delivered within 3-5 business days.
Table of Contents
Companies Mentioned
- Dassault Systèmes SE
- Certara Inc.
- Insilico Medicine
- GNS Healthcare Inc.
- The AnyLogic Company
- Novadiscovery SAS
- InSilicoTrials Technologies SpA
- Immunetrics Inc.
- CATO SMS
- Evotec SE
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 350 |
| Published | July 2025 |
| Forecast Period | 2025 - 2034 |
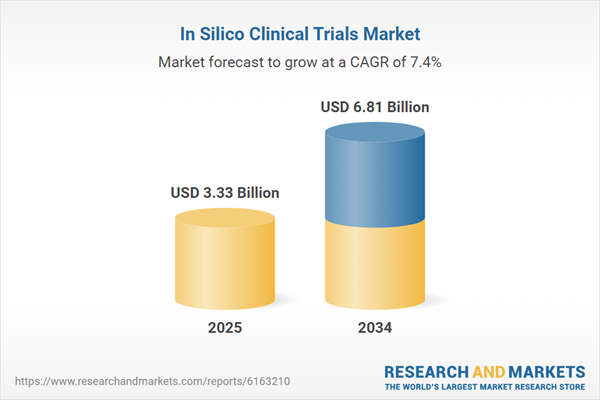
| Estimated Market Value ( USD | $ 3.33 Billion |
| Forecasted Market Value ( USD | $ 6.81 Billion |
| Compound Annual Growth Rate | 7.4% |
| Regions Covered | Global |
| No. of Companies Mentioned | 10 |