High Investment of CDMOs in Advancing Viral-vector Manufacturing and Research in Gene Therapies
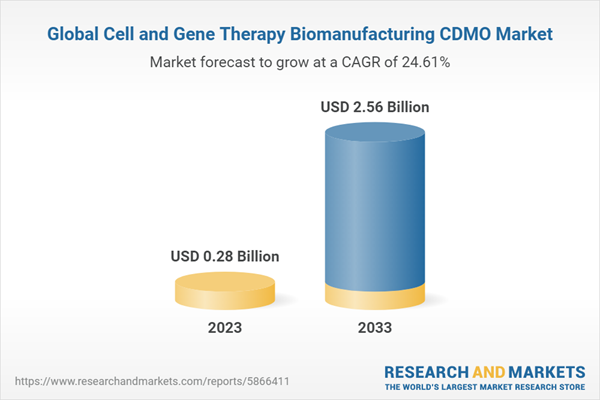
The global cell and gene therapy biomanufacturing CDMO market - focused on AAV was valued at $0.2313 billion in 2022 and is anticipated to reach $2.566 billion by 2033, witnessing a CAGR of 24.61% during the forecast period 2023-2033. The growth in the global cell and gene therapy biomanufacturing CDMO market- focused on AAV is expected to be driven by the rise in clinical activities around viral-vector based gene therapies and high investments of CDMOs in advancing viral-vector manufacturing and research in gene therapies.
Market Lifecycle Stage
The global cell and gene therapy biomanufacturing CDMO market- focused on AAV is currently in a progressing phase. AAV-based gene therapies have gained significant attention and research interest, leading to a higher demand for CDMOs in this market. This increased interest is mainly driven by the potential of AAV-based therapies to treat various genetic disorders and diseases.
Furthermore, partnerships and collaborations between therapy developers and CDMOs play a crucial role in accelerating the availability of cutting-edge AAV-based therapies. By working together, cell and gene therapy developers can focus on their research and clinical activities while outsourcing the manufacturing aspects to CDMOs. This approach enables faster production and time-to-market for these innovative therapies. Additionally, substantial investments in research and development have further fueled advancements in this area, encouraging the exploration of new therapeutic possibilities.
Despite the positive outlook, the global cell and gene therapy biomanufacturing CDMO market- focused on AAV also faces some challenges. One significant challenge is ensuring scalability and maintaining strict quality control standards. As the demand for AAV-based therapies increases, CDMOs need to adapt their manufacturing processes to handle larger production volumes without compromising product quality.
Moreover, the market is becoming more competitive as more CDMOs enter the space to meet the growing demand. This intensified competition could lead to price pressures and potential reductions in profit margins, which may impact the business viability of CDMOs.
Furthermore, the market offers substantial opportunities for all involved parties. Collaborations between CDMOs and therapy developers can drive advancements in AAV biomanufacturing facilities, leading to more efficient and cost-effective production processes. These advancements can further contribute to the overall growth of the market.
Impact
The impact on the global cell and gene therapy biomanufacturing CDMO market- focused on AAV, would be influenced by several factors such as the growing attention and research activities surrounding AAV-based gene therapies would lead to a surge in demand for CDMOs with expertise in AAV biomanufacturing. This increased demand would create more business opportunities for existing CDMOs and attract new players to enter the market. Moreover, the collaborations between therapy developers and CDMOs would foster faster commercialization of AAV-based therapies. This would not only benefit therapy developers by accelerating their time-to-market but also boost the growth of CDMOs, as they establish themselves as reliable partners in the industry. This increasing number of CDMOs entering the market to cater to the rising demand might intensify competition. This could potentially lead to price pressures, as CDMOs may compete to offer competitive rates to secure contracts with therapy developers.
In addition, advancements in AAV biomanufacturing technology could lead to more efficient and cost-effective production processes. CDMOs that adopt these technological innovations would have a competitive advantage, attracting more clients and expanding their market share. However, ensuring scalability in AAV biomanufacturing would be a significant challenge for CDMOs. The ability to handle larger production volumes while maintaining high-quality standards is crucial to meeting the growing demand. CDMOs that can overcome these challenges effectively would be in a better position to thrive in the market.
Impact of COVID-19
The COVID-19 pandemic has had both major and minor impacts on the cell and gene therapy biomanufacturing CDMO market with a focus on AAV.
Operational disruptions caused by lockdowns, restrictions, and supply chain challenges led to delays in manufacturing processes and project timelines. Moreover, clinical trials involving AAV-based therapies also faced disruptions due to enrollment challenges, site closures, and regulatory hurdles, affecting CDMOs involved in providing services in these trials. The pandemic has highlighted the importance of adaptability and resilience in the biomanufacturing sector and as the situation stabilizes, the industry is expected to recover and continue driving advancements in AAV-based therapies.
The uncertain economic environment resulted in cautious funding and investment decisions, impacting the availability of financial resources for CDMOs, and potentially slowing down their expansion plans and technological advancements. In addition, regulatory processes experienced delays and changes as regulatory agencies, including the FDA, redirected resources to address pandemic-related concerns. This could have affected the approval timelines for AAV-based therapies and the manufacturing activities associated with them.
Market Segmentation
Segmentation 1: by Phase of Development
- Clinical Phases I
- Clinical Phases II
- Clinical Phases III
- Commercial Phase
Commercial Phases to Continue its Dominance in the Phase of Development Segment
Based on phase of development, the commercial phase segment dominated the global cell and gene therapy biomanufacturing CDMO market- focused on AAV in FY2022. As more AAV-based therapies continue to advance through clinical phases and gain approval, the commercial phase segment is likely to remain a key driver of growth in the CDMO market.
Segmentation 2: by Workflow
- Upstream Processing
- Downstream Processing
- Formulation, Fill & Finish
Downstream Processing to Continue its Dominance in the Workflow Segment
Based on workflow, the downstream processing segment dominated the global cell and gene therapy biomanufacturing CDMO market- focused on AAV in FY2022. The downstream processing segment encompasses the steps involved after the initial cell culture and vector production, where the focus is on purification, filtration, and isolation of the therapeutic AAV vectors.
Segmentation 3: by Indication
- Oncology
- Ophthalmology
- Infectious Diseases
- Metabolic Disorder
- Neurological Disorder
- Other
Oncology to Continue its Dominance in the Indication Segment
Based on indication, the oncology segment dominated the global cell and gene therapy biomanufacturing CDMO market- focused on AAV in FY2022. The dominance of the oncology segment can be attributed to several factors. Cancer continues to be a significant global health challenge, with a high incidence rate in various regions. The prevalence of cancer and the unmet medical need for effective treatments drove increased research and development in oncology-focused cell and gene therapies
Segmentation 4: by Culture Type
- Adherent Culture
- Suspension Culture
Adherent Culture to Continue its Dominance in the Culture Type Segment
Based on culture type, the adherent culture segment segment dominated the global cell and gene therapy biomanufacturing CDMO market- focused on AAV in FY2022. Adherent cell cultures often yield higher cell densities and higher productivity compared to other culture types. This increased productivity is crucial for generating sufficient quantities of AAV vectors needed for commercial production.
Segmentation 5: by Region
- North America
- Europe
- Asia-Pacific
- Latin America
- Middle East and Africa
North America cell and gene therapy biomanufacturing CDMO market- focused on AAV is expected to have a market share value of 40.92% in 2022 and is currently the leading contributor to the market. However, the Asia-Pacific region, constituting several emerging economies, is expected to register the highest CAGR of 26.66% during the forecast period, 2023-2033.
Recent Developments in the Global Cell and Gene Therapy Biomanufacturing CDMO Market- Focused on AAV
- In March 2023, Remedium Bio and Biovian Oy collaborated agreement to work together on the development of Remedium's innovative disease modifying AAV gene therapy for Osteoarthritis. This agreement aims to advance the research and production of groundbreaking therapy, potentially offering new treatment options for patients with Osteoarthritis.
- In March 2023, Porton Advanced Solutions Ltd. collaborated with DanausGT Biotechnology Co., Ltd. to expedite gene and cell therapy pipelines and develop innovative therapeutics. Porton Advanced, an end-to-end gene and cell therapy service provider, offered comprehensive solutions to DanausGT, including plasmids, viruses, and cell therapy products. The collaboration combined Porton Advanced's expertise with DanausGT's proprietary CRISPR/AAV technology, accelerating the development of cutting-edge Cell and Gene Therapy (CGT) therapeutics.
- In May 2023, Life Biosciences collaborated with Forge Biologics to advance the development of gene therapies for aging-related diseases. Forge Biologics provided AAV process development, toxicology, cGMP manufacturing, and analytical services using their proprietary platform processes. The collaboration aimed to accelerate the production and delivery of innovative gene therapies for these diseases.
- In May 2023, AGC Biologics introduced its BravoAAV viral vector platforms, which provided rapid, efficient, and consistent clinical and commercial GMP production and release. These platforms capitalized on AGC Biologics' extensive experience in Adeno-Associated Viral vector (AAV) development, manufacturing, and analytical expertise accumulated over three decades.
- In March 2023, Charles River Laboratories Inc., launched a Helper Plasmid to streamline the manufacturing process of AAV vectors. This development aimed to enhance efficiency and simplify the production of AAV vectors, a crucial component in gene therapy manufacturing.
Demand - Drivers, Restraints and Opportunities
Market Demand Drivers:
- Rise in Preclinical and Clinical Activities around Viral-Vector Based Gene Therapies Propel the Demand for CDMOs in the Market
- Increasing Number of Emerging Players in the Cell and Gene Therapy (CGT) Sector Upsurge the Demand for CDMOs Services
- High Investment of CDMOs in Advancing Viral-vector Manufacturing and Research in Gene Therapies
Market Restraints:
- Limitations in Development of Analytical Methods for Large-scale Adeno-associated virus (AAV) Biomanufacturing
- High Cost of Viral-vector Manufacturing Creates a Challenge for Emerging CDMOs player in the Market
Market Opportunities:
- Development in the Advancements of AAV Biomanufacturing Facilities by Strategic Collaborations of CDMO Services
How can this report add value to an organization?
Workflow/Innovation Strategy: The cell and gene therapy biomanufacturing CDMO market- focused on AAV (by clinical phases) comprises clinical phase I, clinical phase II, clinical phase III, and commercial phase. Moreover, the study provides the reader with a detailed understanding of the different stages involved in workflow for manufacturing of AAV-based therapies such as upstream processing, downstream processing, and formulation fill & finish.
Growth/Marketing Strategy: Emphasize the CDMO's specialization in AAV-based biomanufacturing for cell and gene therapies. The strategic collaborations with therapy developers, academic institutions, and research organizations to be involved in the early stages of gene therapy development. These partnerships can facilitate long-term relationships and secure a pipeline of projects. In addition, Maintain rigorous quality control standards and ensure compliance with global regulatory requirements.
Competitive Strategy: Key players in the global cell and gene therapy biomanufacturing CDMO market - focused on AAV have been analyzed and profiled in the study, including manufacturers involved in new product launches, acquisitions, expansions, and strategic collaborations. Moreover, a detailed competitive benchmarking of the players operating in the global cell and gene therapy biomanufacturing CDMO market - focused on AAV has been done to help the reader understand how players stack against each other, presenting a clear market landscape. Additionally, comprehensive competitive strategies such as partnerships, agreements, and collaborations will aid the reader in understanding the untapped revenue pockets in the market.
Key Market Players and Competition Synopsis
The global market for cell and gene therapy biomanufacturing CDMO market- focused on AAV is experiencing substantial growth in coming years. The AAV-based gene therapies have shown significant potential in treating various diseases, leading to a high demand for CDMOs specialized in manufacturing these therapies. Collaborations between therapy developers and CDMOs are expected to accelerate the availability of these groundbreaking treatments, ultimately transforming patient care and revolutionizing treatment of genetic disorders and other serious conditions. As the market evolves, CDMOs must continue to invest in cutting-edge technologies, quality systems, and regulatory compliance to meet the increasing demand for AAV-based cell and gene therapies. The high demand for the therapies is driving the growth of cell and gene therapy CDMO biomanufacturers- focused on AAV and creating ample opportunities for them to expand in various geographies and strengthen their market presence.
Key Companies Profiled:
- AGC Biologics.
- Charles River Laboratories International, Inc.
- Catalent, Inc.
- Creative Biogene
- Danaher. (Cytiva)
- FUJIFILM Diosynth Biotechnologies
- Genscript Biotech Corporation (GenScript ProBio)
- Lonza.
- Merck KGaA
- Porton Advanced Solution Ltd.
- PackGene Biotech
- Oxford Biomedica plc
- Thermo Fisher Scientific Inc. (Patheon Pharma Services)
- WuXi AppTec
Table of Contents
Companies Mentioned
- AGC Biologics.
- Charles River Laboratories International, Inc.
- Catalent, Inc.
- Creative Biogene
- Danaher. (Cytiva)
- FUJIFILM Diosynth Biotechnologies
- Genscript Biotech Corporation (GenScript ProBio)
- Lonza.
- Merck KGaA
- Porton Advanced Solution Ltd.
- PackGene Biotech
- Oxford Biomedica plc
- Thermo Fisher Scientific Inc. (Patheon Pharma Services)
- WuXi AppTec
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 336 |
| Published | August 2023 |
| Forecast Period | 2023 - 2033 |
| Estimated Market Value ( USD | $ 0.28 Billion |
| Forecasted Market Value ( USD | $ 2.56 Billion |
| Compound Annual Growth Rate | 24.6% |
| Regions Covered | Global |
| No. of Companies Mentioned | 14 |