Speak directly to the analyst to clarify any post sales queries you may have.
10% Free customizationThis report comes with 10% free customization, enabling you to add data that meets your specific business needs.
Key Market Drivers
Increased Investment in Biopharmaceutical R&D
The market is primarily driven by the surge in investment toward gene therapy research and development. Both viral vectors and plasmid DNA are integral to gene therapy products, which are gaining traction as promising treatments for rare and genetic diseases. These innovations have attracted significant funding from both public and private sectors.As biopharmaceutical companies continue to expand their R&D budgets, there is a clear strategic emphasis on advanced therapies such as gene and cell therapies and DNA-based vaccines. In 2023, the top 20 global pharmaceutical firms - including Swiss leaders Novartis and Roche - collectively invested USD 145 billion in R&D, marking a 4.5% year-over-year increase. This sustained investment underscores a commitment to pipeline expansion and innovation-led growth, heavily reliant on viral vectors and plasmid DNA for therapeutic delivery.
Consequently, the rising number of research programs and preclinical studies is driving robust demand for high-quality viral vectors and plasmids. Manufacturers and suppliers are witnessing increased order volumes, more contract development opportunities, and the formation of long-term strategic partnerships.
Key Market Challenges
Cost and Pricing Pressures
The production of viral vectors and plasmid DNA is technically complex, involving multiple stages and specialized materials and equipment. As gene therapy moves from the research phase to commercial-scale manufacturing, companies face significant challenges in scaling up operations while maintaining stringent quality standards.Achieving cost efficiencies without compromising product integrity is a persistent concern. In addition, compliance with strict regulatory frameworks necessitates investments in quality assurance, documentation, and control systems, adding to operational costs.
Establishing and maintaining GMP-compliant manufacturing facilities and cleanrooms requires substantial capital investment. Moreover, fluctuations in the prices of key raw materials - such as cell culture media, growth factors, and purification reagents - can further elevate production costs. Regulatory requirements for the safe disposal of biological and hazardous waste also contribute to financial pressure.
Key Market Trends
Expansion of the Cell and Gene Therapy Ecosystem
The development of the cell and gene therapy ecosystem is being propelled by collaborative efforts among academic institutions, research centers, and biopharmaceutical companies. These partnerships facilitate knowledge sharing, innovation, and resource pooling, accelerating the advancement of next-generation therapies.Several countries have established dedicated research hubs and institutes focused on cell and gene therapy, providing infrastructure and a collaborative environment for scientific innovation. Notable biotech clusters - such as the Boston-Cambridge corridor in the United States and the Golden Triangle in the United Kingdom - serve as innovation centers, drawing investment and talent. Furthermore, increased funding and grant initiatives from both governmental and private sources continue to support the growth and maturation of the sector, reinforcing its long-term potential.
Key Market Players
- Oxford Biomedica PLC
- Cognate BioServices Inc.
- Cell and Gene Therapy Catapult Ltd.
- FinVector Vision Therapies
- Fujifilm Holdings Corporation (Fujifilm Diosynth Biotechnologies)
- SIRION Biotech GmbH
- Merck KGaA
- Thermo Fisher Scientific Inc.
- Uniqure NV
- Catalent Inc.
Report Scope:
In this report, the Global Viral Vector and Plasmid DNA Manufacturing Market has been segmented into the following categories, in addition to the industry trends which have also been detailed below:Viral Vector and Plasmid DNA Manufacturing Market, By Product Type:
- Plasmid DNA
- Viral Vector
- Non-viral Vector
Viral Vector and Plasmid DNA Manufacturing Market, By Application:
- Cancer
- Genetic Disorder
- Infectious Disease
- Other
Viral Vector and Plasmid DNA Manufacturing Market, By region:
- North America
- United States
- Canada
- Mexico
- Asia-Pacific
- China
- India
- South Korea
- Australia
- Japan
- Europe
- Germany
- France
- United Kingdom
- Spain
- Italy
- South America
- Brazil
- Argentina
- Colombia
- Middle East & Africa
- South Africa
- Saudi Arabia
- UAE
Competitive Landscape
Company Profiles: Detailed analysis of the major companies present in the Global Viral Vector and Plasmid DNA Manufacturing Market.Available Customizations:
With the given market data, the publisher offers customizations according to a company's specific needs. The following customization options are available for the report.Company Information
- Detailed analysis and profiling of additional market players (up to five).
This product will be delivered within 1-3 business days.
Table of Contents
Companies Mentioned
- Oxford Biomedica PLC
- Cognate BioServices Inc.
- Cell and Gene Therapy Catapult Ltd.
- FinVector Vision Therapies
- Fujifilm Holdings Corporation (Fujifilm Diosynth Biotechnologies)
- SIRION Biotech GmbH
- Merck KGaA
- Thermo Fisher Scientific Inc.
- Uniqure NV
- Catalent Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 182 |
| Published | April 2025 |
| Forecast Period | 2024 - 2030 |
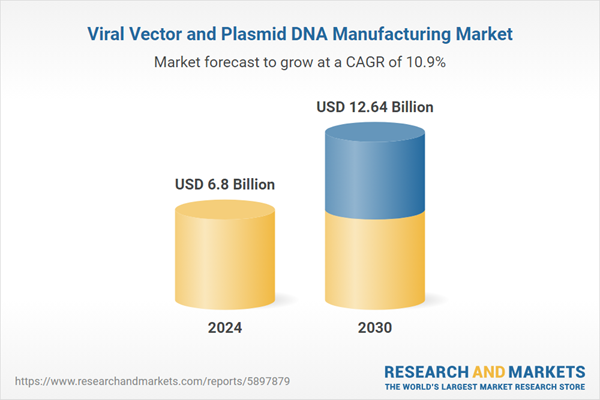
| Estimated Market Value ( USD | $ 6.8 Billion |
| Forecasted Market Value ( USD | $ 12.64 Billion |
| Compound Annual Growth Rate | 10.8% |
| Regions Covered | Global |
| No. of Companies Mentioned | 10 |