Speak directly to the analyst to clarify any post sales queries you may have.
10% Free customizationThis report comes with 10% free customization, enabling you to add data that meets your specific business needs.
Patients receiving nuclear medicine therapies are carefully monitored during and after treatment to assess treatment response and manage potential side effects. Follow-up imaging and tests may be performed to track the progress of the therapy and the patient's condition. Nuclear medicine therapeutics can be tailored to individual patients based on factors such as the type and stage of disease, the patient's overall health, and the response to treatment. This personalized approach aims to maximize treatment efficacy while minimizing side effects.
The global prevalence of cancer continues to increase, driving the demand for effective diagnostic and therapeutic tools. Nuclear medicine plays a crucial role in both cancer diagnosis and treatment, making it a critical component of cancer care. Advances in imaging technologies, such as PET-CT and SPECT-CT, have improved the accuracy of diagnosis and treatment planning. The development of more efficient and compact imaging devices has enhanced the accessibility of nuclear medicine. Ongoing clinical trials and research studies have contributed to the expansion of nuclear medicine applications. Positive trial outcomes can lead to the adoption of nuclear medicine therapies in routine clinical practice.
The aging global population is more susceptible to chronic diseases, including cancer and cardiovascular conditions, which are often diagnosed and treated using nuclear medicine techniques. According to data from the National Institute for Health Research (NIHR) in November 2023, radiopharmaceuticals have been categorized by their development stage (Phase 1/2, 2, and Phase 2/3, 3). The majority (401, 62%) were in Phase 2, while nearly a quarter (146, ~23%) were in Phase 3 clinical trials.
Key Market Drivers
Advancements in Technology
Technological advancements in nuclear medicine therapeutics have been instrumental in improving the accuracy, effectiveness, and safety of diagnostic and treatment procedures. These advancements have expanded the applications of nuclear medicine and enhanced patient care. Hybrid imaging systems, such as PET-CT (Positron Emission Tomography-Computed Tomography) and SPECT-CT (Single-Photon Emission Computed Tomography-Computed Tomography), have become standard in nuclear medicine. These systems combine functional imaging (PET or SPECT) with anatomical imaging (CT) to provide detailed information about both structure and function in a single scan. This allows for more accurate localization of abnormalities and better treatment planning.PET-MRI combines the functional imaging capabilities of PET with the excellent soft tissue contrast of MRI. This technology is particularly valuable in brain imaging and certain oncology applications, offering improved diagnostic accuracy. Research and development efforts have led to the creation of more targeted and effective radiopharmaceuticals. These radiopharmaceuticals can specifically bind to disease markers or receptors, allowing for more precise diagnosis and targeted therapy. Radioligand therapies involve the use of radiopharmaceuticals that target specific receptors on cancer cells. This approach has shown promise in the treatment of various cancers, such as prostate cancer, using agents like Lutetium-177-PSMA.
Advances in alpha-particle-emitting radiopharmaceuticals have gained attention for their high energy and short range, making them effective in targeting cancer cells while sparing surrounding healthy tissue. This approach is being explored for certain types of cancer therapy. Theranostics is an emerging field that combines diagnostic and therapeutic capabilities. It involves the use of radiopharmaceuticals for both imaging and therapy. This approach allows for patient-specific treatment planning based on imaging findings.
For instance, In December 2023, Lantheus Holdings, Inc. and POINT Biopharma Global Inc. (POINT) announced topline results from the Phase 3 SPLASH study, evaluating the efficacy and safety of 177Lu-PNT2002, a PSMA-targeted radioligand therapy for metastatic castration-resistant prostate cancer (mCRPC) after androgen receptor pathway inhibitor (ARPI) treatment. Increasing research investments are expected to drive market growth.
Key Market Challenges
Supply Chain and Radioisotope Availability
Nuclear medicine heavily relies on radioisotopes, which are radioactive materials used in imaging and therapy. The production of these radioisotopes can be complex and is often centralized in a few facilities globally. Any disruptions in production or supply can have widespread implications for nuclear medicine services. Many radioisotopes used in nuclear medicine have short half-lives, meaning they decay rapidly. This necessitates just-in-time production and delivery to healthcare facilities. Any delays in production, transportation, or delivery can lead to radioisotope shortages.The global supply chain for radioisotopes can be vulnerable to various factors, such as technical issues in production facilities, regulatory challenges, geopolitical tensions, and transportation disruptions. These vulnerabilities can lead to interruptions in the supply of radioisotopes. The demand for radioisotopes, especially those used in oncology and cardiology, has been steadily increasing. Meeting this growing demand while ensuring a stable supply chain can be a logistical challenge. There are relatively few facilities worldwide that produce medical-grade radioisotopes.
Relying on a small number of production centers increases the risk of supply disruptions. The production of radioisotopes often involves the use of nuclear reactors or particle accelerators, which require specialized infrastructure and expertise. Technical challenges or maintenance shutdowns can affect production. Radioisotopes must be transported under strict safety regulations. Delays or disruptions in transportation can impact the timely delivery of radiopharmaceuticals to healthcare facilities.
Key Market Trends
Radiopharmaceutical Production
The growing awareness of nuclear medicine's diagnostic and therapeutic capabilities has led to an increased demand for radiopharmaceuticals. This trend is driven by the rising prevalence of diseases such as cancer and cardiovascular conditions that benefit from nuclear medicine procedures. Ongoing research and development efforts have led to the creation of a diverse range of radiopharmaceuticals, including those for PET, SPECT, and therapeutic applications. This diversification allows for a broader spectrum of medical conditions to be addressed using nuclear medicine. Radiopharmaceuticals can be designed to target specific disease markers or receptors, enabling more precise diagnosis and therapy.Targeted radiopharmaceuticals are a growing area of interest, particularly in oncology. The concept of theranostics, which combines diagnostic and therapeutic capabilities using the same radiopharmaceutical, has gained traction. Theranostic approaches allow for personalized treatment planning based on diagnostic imaging findings. Improvements in radioisotope production methods have contributed to a more reliable and efficient supply of radiopharmaceuticals. Cyclotron and generator technologies have advanced to support increased production capacity. Efforts have been made to reduce the production time for radiopharmaceuticals with short half-lives. This is crucial for ensuring timely access to these materials.
Key Market Players
- Actinium Pharmaceutical Inc.
- Alpha Tau Medical Ltd
- Bayer AG
- Fusion Pharmaceuticals Inc.
- IBA Radiopharma Solutions
- RadioMedix Inc.
- Telix Pharmaceuticals Ltd
- NTP Radioisotopes Pty Ltd.
- Bracco SpA
- Cardinal Health Inc.
- Nordion Inc. (Sotera Health Company)
- Triad Isotopes Inc. (Jubilant Life Sciences)
Report Scope:
In this report, the Global Nuclear Medicine Therapeutics Market has been segmented into the following categories, in addition to the industry trends which have also been detailed below:Nuclear Medicine Therapeutics Market, By Type:
- Alpha Emitters
- Radium-223 (RA-223) & Alpharadin
- Actinium-225 (AC-225)
- Lead-212 (PB-212)/Bismuth-212 (BI-212)
- Other Alpha Emitters
- Beta Emitters
- Iodine-131 (I-131)
- Yttrium-90 (Y-90)
- Other Beta Emitters
- Brachytherapy
- Cesium-131
- Iodine-125
- Other Brachytherapies
Nuclear Medicine Therapeutics Market, By Application:
- Oncology
- Cardiology
- Thyroid
- Other Applications
Nuclear Medicine Therapeutics Market, By Region:
- North America
- United States
- Canada
- Mexico
- Asia-Pacific
- China
- India
- South Korea
- Australia
- Japan
- Europe
- Germany
- France
- United Kingdom
- Spain
- Italy
- South America
- Brazil
- Argentina
- Colombia
- Middle East & Africa
- South Africa
- Saudi Arabia
- UAE
Competitive Landscape
Company Profiles: Detailed analysis of the major companies present in the Global Nuclear Medicine Therapeutics Market.Available Customizations:
With the given market data, the publisher offers customizations according to a company's specific needs. The following customization options are available for the report.Company Information
- Detailed analysis and profiling of additional market players (up to five).
This product will be delivered within 1-3 business days.
Table of Contents
Companies Mentioned
- Actinium Pharmaceutical Inc.
- Alpha Tau Medical Ltd
- Bayer AG
- Fusion Pharmaceuticals Inc.
- IBA Radiopharma Solutions
- RadioMedix Inc.
- Telix Pharmaceuticals Ltd
- NTP Radioisotopes Pty Ltd.
- Bracco SpA
- Cardinal Health Inc.
- Nordion Inc. (Sotera Health Company)
- Triad Isotopes Inc. (Jubilant Life Sciences)
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 180 |
| Published | March 2025 |
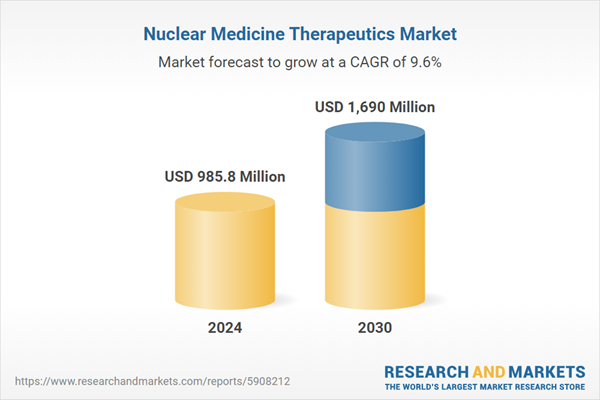
| Forecast Period | 2024 - 2030 |
| Estimated Market Value ( USD | $ 985.8 Million |
| Forecasted Market Value ( USD | $ 1690 Million |
| Compound Annual Growth Rate | 9.6% |
| Regions Covered | Global |
| No. of Companies Mentioned | 12 |