Speak directly to the analyst to clarify any post sales queries you may have.
10% Free customizationThis report comes with 10% free customization, enabling you to add data that meets your specific business needs.
These blood diseases are not present at birth or inherited through genetics. Instead, they develop or are acquired during a person's life, often due to various triggers or underlying health conditions. Diagnosis, treatment, and management of acquired orphan blood diseases often require specialized care from hematologists or other healthcare providers with expertise in rare blood disorders. Treatment approaches may include blood transfusions, immunosuppressive therapies, targeted therapies, and, in some cases, bone marrow transplantation.
Ongoing advancements in medical research, including genomics, immunology, and molecular biology, have deepened our understanding of the pathogenesis of these diseases. This knowledge supports the development of targeted therapies and treatment innovations. Regulatory incentives provided by orphan drug designations, such as extended market exclusivity, tax credits, and reduced development costs, encourage pharmaceutical companies to invest in research and development for rare diseases. Patient advocacy groups and organizations play a pivotal role in driving research, raising awareness, and advocating for improved access to treatments.
Their efforts help accelerate progress in the field of acquired orphan blood diseases. The emergence of novel treatment modalities, including gene therapies, monoclonal antibodies, and immunomodulatory agents, has expanded the range of therapeutic options available for acquired orphan blood diseases. Advances in diagnostic techniques and technologies have led to more accurate and timely diagnoses of acquired orphan blood diseases. This enables early intervention and treatment.
Key Market Drivers
Advancements in Medical Research
Medical research has deepened our understanding of the underlying mechanisms and pathophysiology of acquired orphan blood diseases. This knowledge helps identify novel therapeutic targets and pathways for intervention. For instance, in February 2025, a new 3D lung model will revolutionize respiratory medicine research. This advanced model replicates lung structure and function, enabling more accurate drug testing and disease modeling. It offers a powerful tool for studying conditions like asthma and COPD, accelerating the development of innovative treatments and improving patient outcomes.Research efforts have led to the development of targeted therapies that address the specific molecular and cellular abnormalities associated with these diseases. Targeted treatments often offer better efficacy and safety profiles compared to traditional therapies. Research has contributed to the identification of biomarkers and genetic markers associated with these diseases. Biomarkers enable early diagnosis, disease monitoring, and personalized treatment approaches. Ongoing research supports drug discovery and development programs aimed at creating innovative and more effective therapeutics.
This includes the development of monoclonal antibodies, gene therapies, and small molecule drugs. Advances in clinical trial design and methodology, including adaptive trials and innovative endpoints, facilitate the evaluation of potential therapies. Clinical trials are essential for assessing treatment safety and efficacy. Medical research has led to the creation of patient registries and databases for rare diseases. These registries facilitate data collection, epidemiological studies, and clinical trial recruitment.
Key Market Challenges
Access to Treatment
Rare diseases, including acquired orphan blood diseases, often have a limited number of treatment options available due to their low prevalence. This scarcity of therapies can make it difficult for patients to access appropriate treatments. Many orphan drugs and emerging therapies are associated with high treatment costs. The rarity of these diseases and the costs of research and development often result in expensive therapies, making them financially burdensome for patients and healthcare systems. Acquired orphan blood diseases are not always well-known among healthcare providers and the public. This lack of awareness can lead to delayed diagnoses and difficulty in accessing specialized treatments.Access to advanced medical treatments can vary significantly based on geographic location. Patients in remote or underserved areas may face challenges in accessing specialized healthcare facilities and therapies. In some regions, the healthcare infrastructure may not be adequately equipped to diagnose and manage acquired orphan blood diseases. This can lead to delays in treatment initiation.
The availability and extent of insurance coverage for orphan blood disease therapies can vary widely. In some cases, insurance plans may not fully cover the cost of treatment, leaving patients with high out-of-pocket expenses. Pharmaceutical companies may encounter challenges in obtaining reimbursement approvals for orphan drugs, leading to delays in patients' access to treatment. Specialized treatment centers and healthcare providers with expertise in acquired orphan blood diseases may be concentrated in urban areas, making it challenging for patients in rural regions to access care.
Key Market Trends
Expanded Access Programs
(Expanded access programs) EAPs prioritize the needs of patients who have limited or no alternative treatment options. These programs provide access to potentially life-saving therapies, reflecting a patient-centered approach to healthcare. EAPs allow patients to access investigational or emerging therapies that are still in the clinical trial or regulatory approval phases. This can be especially valuable for patients with rare and life-threatening diseases like acquired orphan blood diseases. EAPs are driven by ethical considerations, recognizing the urgency of providing treatments to patients who may not qualify for clinical trials or cannot wait for regulatory approvals. They provide hope and potential benefits to those in need. Patients who do not respond to standard therapies or who have contraindications to traditional treatments may find EAPs as a viable option for accessing novel treatments tailored to their specific condition. EAPs allow for the collection of real-world data and evidence regarding the safety and efficacy of therapies in diverse patient populations. This information can complement clinical trial data and inform treatment decisions. EAPs involve close collaboration between physicians, patients, pharmaceutical companies, and regulatory authorities. This collaboration ensures that patients receive the most appropriate and personalized care.Key Market Players
- Alexion Pharmaceuticals, Inc.
- Amgen, Inc.
- Celgene Corporation
- Eli Lilly and Company
- Sanofi S.A.
- GlaxoSmithKline plc,
- Cyclacel Pharmaceuticals, Inc.
- Onconova Therapeutics, Inc.
- Incyte Corporation,
- CTI BioPharma Corp
Report Scope:
In this report, the Global Acquired Orphan Blood Diseases Therapeutics Market has been segmented into the following categories, in addition to the industry trends which have also been detailed below:Acquired Orphan Blood Diseases Therapeutics Market, By Therapy:
- Recombinant Factor
- Immunoglobulin Infusion Therapy
- Activated Prothrombin Complex Concentrate
- Thrombopoietin Receptor Agonists
- Others
Acquired Orphan Blood Diseases Therapeutics Market, By Disease Indication:
- Acquired Agranulocytosis
- Acquired Hemophilia
- Acquired Von Willebrand Syndrome
- Paroxysmal Nocturnal Hemoglobinuria (PNH)
- Myelodysplastic Syndrome
- Others
Acquired Orphan Blood Diseases Therapeutics Market, By Distribution Channel:
- Hospital Pharmacy
- Retail Pharmacy
- Others
Acquired Orphan Blood Diseases Therapeutics Market, By region:
- North America
- United States
- Canada
- Mexico
- Asia-Pacific
- China
- India
- South Korea
- Australia
- Japan
- Europe
- Germany
- France
- United Kingdom
- Spain
- Italy
- South America
- Brazil
- Argentina
- Colombia
- Middle East & Africa
- South Africa
- Saudi Arabia
- UAE
Competitive Landscape
Company Profiles: Detailed analysis of the major companies present in the Global Acquired Orphan Blood Diseases Therapeutics Market.Available Customizations:
With the given market data, the publisher offers customizations according to a company's specific needs. The following customization options are available for the report.Company Information
- Detailed analysis and profiling of additional market players (up to five).
This product will be delivered within 1-3 business days.
Table of Contents
Companies Mentioned
- Alexion Pharmaceuticals, Inc.
- Amgen, Inc.
- Celgene Corporation
- Eli Lilly and Company
- Sanofi S.A.
- GlaxoSmithKline plc,
- Cyclacel Pharmaceuticals, Inc.
- Onconova Therapeutics, Inc.
- Incyte Corporation,
- CTI BioPharma Corp
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 180 |
| Published | March 2025 |
| Forecast Period | 2024 - 2030 |
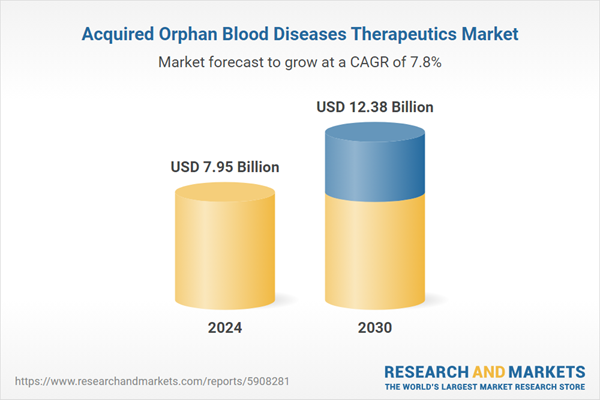
| Estimated Market Value ( USD | $ 7.95 Billion |
| Forecasted Market Value ( USD | $ 12.38 Billion |
| Compound Annual Growth Rate | 7.8% |
| Regions Covered | Global |
| No. of Companies Mentioned | 10 |