Global Acquired Orphan Blood Diseases Therapeutics Market - Key Trends and Drivers Summarized
Acquired orphan blood diseases, which include conditions such as aplastic anemia, paroxysmal nocturnal hemoglobinuria (PNH), and myelodysplastic syndromes (MDS), represent a group of rare hematological disorders. These diseases are characterized by their low prevalence and complexity in both diagnosis and treatment. Therapeutics for these conditions are considered 'orphan drugs' due to the rare nature of the diseases they treat and the lack of financial incentive for private companies to develop treatments without government assistance.Recent advances in the therapeutics for acquired orphan blood diseases have focused on targeted therapies that address specific pathways involved in disease progression. For example, treatments for PNH have transformed with the introduction of complement inhibitors, which help reduce blood cell destruction, improve quality of life, and decrease the need for blood transfusions. In the case of MDS, new drug therapies that modify the disease's epigenetic landscape have shown promise in managing symptoms and extending survival rates.
The growth in the therapeutics market for acquired orphan blood diseases is driven by increased research and development activities, advancements in genetic testing that facilitate early and accurate diagnosis, and a robust pipeline of drugs that hold promise for better disease management. Additionally, government incentives, including extended patent exclusivity and tax benefits, have encouraged pharmaceutical companies to invest in the research of orphan drugs. International collaborations in clinical trials are also expanding, aiming to gather comprehensive data and accelerate the approval process of new therapies. Despite these advancements, challenges such as high treatment costs and limited patient populations continue to impede broader access to these crucial medications, highlighting the need for continued innovation and policy support to improve treatment outcomes in this complex therapeutic area.
Report Scope
The report analyzes the Acquired Orphan Blood Diseases Therapeutics market, presented in terms of market value. The analysis covers the key segments and geographic regions outlined below.- Segments: Therapy Type (Immunoglobulin Infusion Therapy, Recombinant Factor Therapy, Activated Prothrombin Complex Concentrate Therapy, Thrombopoietin Receptor Agonists Therapy, Other Therapy Types); Disease Type (Acquired Hemophilia Disease, Paroxysmal Nocturnal Hemoglobinuria (PNH) Disease, Acquired Agranulocytosis Disease, Acquired Von Willebrand Syndrome Disease, Myelodysplastic Syndrome Disease, Other Disease Types); Distribution Channel (Hospital Pharmacies, Retail Pharmacies, Other Distribution Channels).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Immunoglobulin Infusion Therapy segment, which is expected to reach US$4.6 Billion by 2030 with a CAGR of a 5%. The Recombinant Factor Therapy segment is also set to grow at 7% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $2.4 Billion in 2024, and China, forecasted to grow at an impressive 8.6% CAGR to reach $2.6 Billion by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Acquired Orphan Blood Diseases Therapeutics Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Acquired Orphan Blood Diseases Therapeutics Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Acquired Orphan Blood Diseases Therapeutics Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as Amgen Inc., Apellis Pharmaceuticals, Chartwell Pennsylvania, LP, CSL Limited, F. Hoffmann-La Roche Ltd and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 86 companies featured in this Acquired Orphan Blood Diseases Therapeutics market report include:
- Amgen Inc.
- Apellis Pharmaceuticals
- Chartwell Pennsylvania, LP
- CSL Limited
- F. Hoffmann-La Roche Ltd
- FFF Enterprises, Inc.
- KabaFusion
- Novo Nordisk A/S
- Nufactor Inc.
- Option Care Health Inc.
- Optum, Inc.
- Pfizer Inc.
- Sanofi SA
- Soleo Health
- Takeda Pharmaceutical Company Limited
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Amgen Inc.
- Apellis Pharmaceuticals
- Chartwell Pennsylvania, LP
- CSL Limited
- F. Hoffmann-La Roche Ltd
- FFF Enterprises, Inc.
- KabaFusion
- Novo Nordisk A/S
- Nufactor Inc.
- Option Care Health Inc.
- Optum, Inc.
- Pfizer Inc.
- Sanofi SA
- Soleo Health
- Takeda Pharmaceutical Company Limited
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 434 |
| Published | February 2026 |
| Forecast Period | 2024 - 2030 |
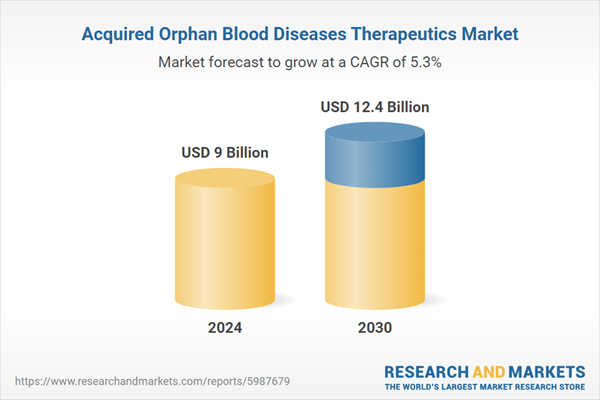
| Estimated Market Value ( USD | $ 9 Billion |
| Forecasted Market Value ( USD | $ 12.4 Billion |
| Compound Annual Growth Rate | 5.3% |
| Regions Covered | Global |