1. Research Methodology
1.1. Study Objectives
1.2. Study Scope
1.3. Research Assumptions
1.4. Research Framework
2. Introduction
2.1. Market Definition
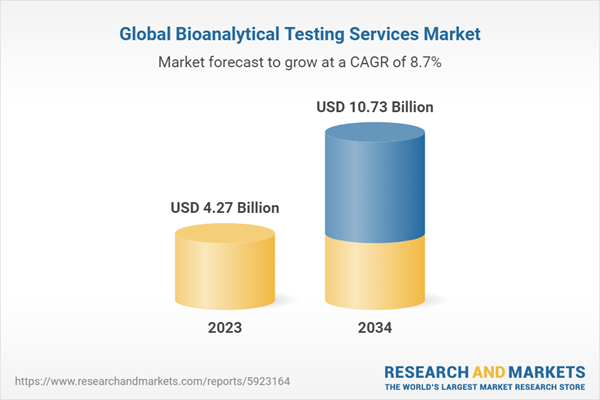
2.2. Global Bioanalytical Testing Services Market Overview
4. Market Environment Analysis
4.1. Porter’s 5 Forces Analysis
4.2. PESTEL Analysis
4.3. SWOT Analysis
5. Market Dynamics
5.1. Drivers Analysis
5.2. Restraints Analysis
5.3. Opportunities Analysis
5.4. Threats Analysis
5.5. Trend Analysis
7. Bioanalytical Testing Services Market: Molecule Estimates & Trend Analysis
7.1. Molecule Segment Opportunity Analysis
7.2. Small Molecule
7.2.1. Small Molecule Market Analysis & Forecast, 2023-2034 (Revenue, USD Bn)
7.3. Large Molecule
7.3.1. Large Molecule Market Analysis & Forecast, 2023-2034 (Revenue, USD Bn)
7.3.2. Immunoassays
7.3.2.1. Immunoassays Market Analysis & Forecast, 2023-2034 (Revenue, USD Bn)
7.3.2.2. ADA
7.3.2.2.1. ADA Market Analysis & Forecast, 2023-2034 (Revenue, USD Bn)
7.3.2.3. PK
7.3.2.3.1. PK Market Analysis & Forecast, 2023-2034 (Revenue, USD Bn)
7.3.2.4. Others
7.3.2.4.1. Others Market Analysis & Forecast, 2023-2034 (Revenue, USD Bn)
7.3.3. LC-MS Studies
7.3.3.1. LC-MS Studies Market Analysis & Forecast, 2023-2034 (Revenue, USD Bn)
7.3.4. Others
7.3.4.1. Others Market Analysis & Forecast, 2023-2034 (Revenue, USD Bn)
8. Bioanalytical Testing Services Market: Workflow Estimates & Trend Analysis
8.1. Workflow Segment Opportunity Analysis
8.2. Sample Analysis
8.2.1. Sample Analysis Market Analysis & Forecast, 2023-2034 (Revenue, USD Bn)
8.2.2. Electrophoresis
8.2.2.1. Electrophoresis Market Analysis & Forecast, 2023-2034 (Revenue, USD Bn)
8.2.3. Mass Spectrometry
8.2.3.1. Mass Spectrometry Market Analysis & Forecast, 2023-2034 (Revenue, USD Bn)
8.2.4. Ligand Binding Assay
8.2.4.1. Ligand Binding Assay Market Analysis & Forecast, 2023-2034 (Revenue, USD Bn)
8.2.5. Hyphenated Technique
8.2.5.1. Hyphenated Technique Market Analysis & Forecast, 2023-2034 (Revenue, USD Bn)
8.2.6. Chromatographic Technique
8.2.6.1. Chromatographic Technique Market Analysis & Forecast, 2023-2034 (Revenue, USD Bn)
8.2.7. Nuclear Magnetic Resonance
8.2.7.1. Nuclear Magnetic Resonance Market Analysis & Forecast, 2023-2034 (Revenue, USD Bn)
8.3. Sample Preparation
8.3.1. Sample Preparation Market Analysis & Forecast, 2023-2034 (Revenue, USD Bn)
8.3.2. Protein Precipitation
8.3.2.1. Protein Precipitation Market Analysis & Forecast, 2023-2034 (Revenue, USD Bn)
8.3.3. Solid Phase Extraction
8.3.3.1. Solid Phase Extraction Market Analysis & Forecast, 2023-2034 (Revenue, USD Bn)
8.3.4. Liquid-Liquid Extraction
8.3.4.1. Liquid-Liquid Extraction Market Analysis & Forecast, 2023-2034 (Revenue, USD Bn)
8.4. Other
8.4.1. Other Market Analysis & Forecast, 2023-2034 (Revenue, USD Bn)
9. Bioanalytical Testing Services Market: Test Estimates & Trend Analysis
9.1. Test Segment Opportunity Analysis
9.2. Absorption Distribution Metabolism Excretion (ADME)
9.2.1. Absorption Distribution Metabolism Excretion (ADME) Market Analysis & Forecast, 2023-2034 (Revenue, USD Bn)
9.2.2. In-Vitro
9.2.2.1. In-Vitro Market Analysis & Forecast, 2023-2034 (Revenue, USD Bn)
9.2.3. In-Vivo
9.2.3.1. In-Vivo Market Analysis & Forecast, 2023-2034 (Revenue, USD Bn)
9.3. Pharmacodynamics (PD)
9.3.1. Pharmacodynamics (PD) Market Analysis & Forecast, 2023-2034 (Revenue, USD Bn)
9.4. Pharmacokinetics (PK)
9.4.1. Pharmacokinetics (PK) Market Analysis & Forecast, 2023-2034 (Revenue, USD Bn)
9.5. Bioequivalence
9.5.1. Bioequivalence Market Analysis & Forecast, 2023-2034 (Revenue, USD Bn)
9.6. Bioavailability
9.6.1. Bioavailability Market Analysis & Forecast, 2023-2034 (Revenue, USD Bn)
9.7. Others
9.7.1. Others Market Analysis & Forecast, 2023-2034 (Revenue, USD Bn)
10. Bioanalytical Testing Services Market: Therapeutic Area Estimates & Trend Analysis
10.1. Therapeutic Area Segment Opportunity Analysis
10.2. Cardiology
10.2.1. Cardiology Market Analysis & Forecast, 2023-2034 (Revenue, USD Bn)
10.3. Immunology
10.3.1. Immunology Market Analysis & Forecast, 2023-2034 (Revenue, USD Bn)
10.4. Neurology
10.4.1. Neurology Market Analysis & Forecast, 2023-2034 (Revenue, USD Bn)
10.5. Orthopedics
10.5.1. Orthopedics Market Analysis & Forecast, 2023-2034 (Revenue, USD Bn)
10.6. Oncology
10.6.1. Oncology Market Analysis & Forecast, 2023-2034 (Revenue, USD Bn)
10.7. Gastroenterology
10.7.1. Gastroenterology Market Analysis & Forecast, 2023-2034 (Revenue, USD Bn)
10.8. Haematology
10.8.1. Haematology Market Analysis & Forecast, 2023-2034 (Revenue, USD Bn)
10.9. Others
10.9.1. Others Market Analysis & Forecast, 2023-2034 (Revenue, USD Bn)
11. Bioanalytical Testing Services Market: End-user Estimates & Trend Analysis
11.1. End-user Segment Opportunity Analysis
11.2. Contract Development & Manufacturing Organization
11.2.1. Contract Development & Manufacturing Organization Market Analysis & Forecast, 2023-2034 (Revenue, USD Bn)
11.3. Pharmaceutical & Biotechnology Companies
11.3.1. Pharmaceutical & Biotechnology Companies Market Analysis & Forecast, 2023-2034 (Revenue, USD Bn)
11.4. Contract Research Organization
11.4.1. Contract Research Organization Market Analysis & Forecast, 2023-2034 (Revenue, USD Bn)
11.5. Others
11.5.1. Others Market Analysis & Forecast, 2023-2034 (Revenue, USD Bn)
12. Regional Market Analysis
12.1. Regional Market Opportunity Analysis
13. North America Bioanalytical Testing Services Market
13.1. North America Bioanalytical Testing Services Market
13.1.1. North America Bioanalytical Testing Services Market Size and Forecast, 2023-2034 (Revenue USD Bn)
13.1.2. North America Bioanalytical Testing Services Market Size and Forecast, By Country, 2023-2034 (Revenue USD Bn)
13.1.3. North America Bioanalytical Testing Services Market Size and Forecast, By Molecule, 2023-2034 (Revenue USD Bn)
13.1.4. North America Bioanalytical Testing Services Market Size and Forecast, By Workflow, 2023-2034 (Revenue USD Bn)
13.1.5. North America Bioanalytical Testing Services Market Size and Forecast, By Test, 2023-2034 (Revenue USD Bn)
13.1.6. North America Bioanalytical Testing Services Market Size and Forecast, By Therapeutic Area, 2023-2034 (Revenue USD Bn)
13.1.7. North America Bioanalytical Testing Services Market Size and Forecast, By End-user, 2023-2034 (Revenue USD Bn)
13.2. U.S. Global Bioanalytical Testing Services Market
13.2.1. U.S. Bioanalytical Testing Services Market Size and Forecast, 2023-2034 (Revenue USD Bn)
13.2.2. U.S. Bioanalytical Testing Services Market Size and Forecast, By Molecule, 2023-2034 (Revenue USD Bn)
13.2.3. U.S. Bioanalytical Testing Services Market Size and Forecast, By Workflow, 2023-2034 (Revenue USD Bn)
13.2.4. U.S. Bioanalytical Testing Services Market Size and Forecast, By Test, 2023-2034 (Revenue USD Bn)
13.2.5. U.S. Bioanalytical Testing Services Market Size and Forecast, By Therapeutic Area, 2023-2034 (Revenue USD Bn)
13.2.6. U.S. Bioanalytical Testing Services Market Size and Forecast, By End-user, 2023-2034 (Revenue USD Bn)
13.3. Canada Global Bioanalytical Testing Services Market
13.3.1. Canada Bioanalytical Testing Services Market Size and Forecast, 2023-2034 (Revenue USD Bn)
13.3.2. Canada Bioanalytical Testing Services Market Size and Forecast, By Molecule, 2023-2034 (Revenue USD Bn)
13.3.3. Canada Bioanalytical Testing Services Market Size and Forecast, By Workflow, 2023-2034 (Revenue USD Bn)
13.3.4. Canada Bioanalytical Testing Services Market Size and Forecast, By Test, 2023-2034 (Revenue USD Bn)
13.3.5. Canada Bioanalytical Testing Services Market Size and Forecast, By Therapeutic Area, 2023-2034 (Revenue USD Bn)
13.3.6. Canada Bioanalytical Testing Services Market Size and Forecast, By End-user, 2023-2034 (Revenue USD Bn)
14. Europe Global Bioanalytical Testing Services Market
14.1. Europe Global Bioanalytical Testing Services Market
14.1.1. Europe Bioanalytical Testing Services Market Size and Forecast, 2023-2034 (Revenue USD Bn)
14.1.2. Europe Bioanalytical Testing Services Market Size and Forecast, By Country, 2023-2034 (Revenue USD Bn)
14.1.3. Europe Bioanalytical Testing Services Market Size and Forecast, By Molecule, 2023-2034 (Revenue USD Bn)
14.1.4. Europe Bioanalytical Testing Services Market Size and Forecast, By Workflow, 2023-2034 (Revenue USD Bn)
14.1.5. Europe Bioanalytical Testing Services Market Size and Forecast, By Test, 2023-2034 (Revenue USD Bn)
14.1.6. Europe Bioanalytical Testing Services Market Size and Forecast, By Therapeutic Area, 2023-2034 (Revenue USD Bn)
14.1.7. Europe Bioanalytical Testing Services Market Size and Forecast, By End-user, 2023-2034 (Revenue USD Bn)
14.2. Germany Global Bioanalytical Testing Services Market
14.2.1. Germany Bioanalytical Testing Services Market Size and Forecast, 2023-2034 (Revenue USD Bn)
14.2.2. Germany Bioanalytical Testing Services Market Size and Forecast, By Molecule, 2023-2034 (Revenue USD Bn)
14.2.3. Germany Bioanalytical Testing Services Market Size and Forecast, By Workflow, 2023-2034 (Revenue USD Bn)
14.2.4. Germany Bioanalytical Testing Services Market Size and Forecast, By Test, 2023-2034 (Revenue USD Bn)
14.2.5. Germany Bioanalytical Testing Services Market Size and Forecast, By Therapeutic Area, 2023-2034 (Revenue USD Bn)
14.2.6. Germany Bioanalytical Testing Services Market Size and Forecast, By End-user, 2023-2034 (Revenue USD Bn)
14.3. UK Global Bioanalytical Testing Services Market
14.3.1. UK Bioanalytical Testing Services Market Size and Forecast, 2023-2034 (Revenue USD Bn)
14.3.2. UK Bioanalytical Testing Services Market Size and Forecast, By Molecule, 2023-2034 (Revenue USD Bn)
14.3.3. UK Bioanalytical Testing Services Market Size and Forecast, By Workflow, 2023-2034 (Revenue USD Bn)
14.3.4. UK Bioanalytical Testing Services Market Size and Forecast, By Test, 2023-2034 (Revenue USD Bn)
14.3.5. UK Bioanalytical Testing Services Market Size and Forecast, By Therapeutic Area, 2023-2034 (Revenue USD Bn)
14.3.6. UK Bioanalytical Testing Services Market Size and Forecast, By End-user, 2023-2034 (Revenue USD Bn)
14.4. France Global Bioanalytical Testing Services Market
14.4.1. France Bioanalytical Testing Services Market Size and Forecast, 2023-2034 (Revenue USD Bn)
14.4.2. France Bioanalytical Testing Services Market Size and Forecast, By Molecule, 2023-2034 (Revenue USD Bn)
14.4.3. France Bioanalytical Testing Services Market Size and Forecast, By Workflow, 2023-2034 (Revenue USD Bn)
14.4.4. France Bioanalytical Testing Services Market Size and Forecast, By Test, 2023-2034 (Revenue USD Bn)
14.4.5. France Bioanalytical Testing Services Market Size and Forecast, By Therapeutic Area, 2023-2034 (Revenue USD Bn)
14.4.6. France Bioanalytical Testing Services Market Size and Forecast, By End-user, 2023-2034 (Revenue USD Bn)
14.5. Spain Global Bioanalytical Testing Services Market
14.5.1. Spain Bioanalytical Testing Services Market Size and Forecast, 2023-2034 (Revenue USD Bn)
14.5.2. Spain Bioanalytical Testing Services Market Size and Forecast, By Molecule, 2023-2034 (Revenue USD Bn)
14.5.3. Spain Bioanalytical Testing Services Market Size and Forecast, By Workflow, 2023-2034 (Revenue USD Bn)
14.5.4. Spain Bioanalytical Testing Services Market Size and Forecast, By Test, 2023-2034 (Revenue USD Bn)
14.5.5. Spain Bioanalytical Testing Services Market Size and Forecast, By Therapeutic Area, 2023-2034 (Revenue USD Bn)
14.5.6. Spain Bioanalytical Testing Services Market Size and Forecast, By End-user, 2023-2034 (Revenue USD Bn)
14.6. Italy Global Bioanalytical Testing Services Market
14.6.1. Italy Bioanalytical Testing Services Market Size and Forecast, 2023-2034 (Revenue USD Bn)
14.6.2. Italy Bioanalytical Testing Services Market Size and Forecast, By Molecule, 2023-2034 (Revenue USD Bn)
14.6.3. Italy Bioanalytical Testing Services Market Size and Forecast, By Workflow, 2023-2034 (Revenue USD Bn)
14.6.4. Italy Bioanalytical Testing Services Market Size and Forecast, By Test, 2023-2034 (Revenue USD Bn)
14.6.5. Italy Bioanalytical Testing Services Market Size and Forecast, By Therapeutic Area, 2023-2034 (Revenue USD Bn)
14.6.6. Italy Bioanalytical Testing Services Market Size and Forecast, By End-user, 2023-2034 (Revenue USD Bn)
14.7. Rest of Europe Global Bioanalytical Testing Services Market
14.7.1. Rest of Europe Bioanalytical Testing Services Market Size and Forecast, 2023-2034 (Revenue USD Bn)
14.7.2. Rest of Europe Bioanalytical Testing Services Market Size and Forecast, By Molecule, 2023-2034 (Revenue USD Bn)
14.7.3. Rest of Europe Bioanalytical Testing Services Market Size and Forecast, By Workflow, 2023-2034 (Revenue USD Bn)
14.7.4. Rest of Europe Bioanalytical Testing Services Market Size and Forecast, By Test, 2023-2034 (Revenue USD Bn)
14.7.5. Rest of Europe Bioanalytical Testing Services Market Size and Forecast, By Therapeutic Area, 2023-2034 (Revenue USD Bn)
14.7.6. Rest of Europe Bioanalytical Testing Services Market Size and Forecast, By End-user, 2023-2034 (Revenue USD Bn)
15. Asia Pacific Global Bioanalytical Testing Services Market
15.1. Asia Pacific Global Bioanalytical Testing Services Market
15.1.1. Asia Pacific Bioanalytical Testing Services Market Size and Forecast, 2023-2034 (Revenue USD Bn)
15.1.2. Asia Pacific Bioanalytical Testing Services Market Size and Forecast, By Country, 2023-2034 (Revenue USD Bn)
15.1.3. Asia Pacific Bioanalytical Testing Services Market Size and Forecast, By Molecule, 2023-2034 (Revenue USD Bn)
15.1.4. Asia Pacific Bioanalytical Testing Services Market Size and Forecast, By Workflow, 2023-2034 (Revenue USD Bn)
15.1.5. Asia Pacific Bioanalytical Testing Services Market Size and Forecast, By Test, 2023-2034 (Revenue USD Bn)
15.1.6. Asia Pacific Bioanalytical Testing Services Market Size and Forecast, By Therapeutic Area, 2023-2034 (Revenue USD Bn)
15.1.7. Asia Pacific Bioanalytical Testing Services Market Size and Forecast, By End-user, 2023-2034 (Revenue USD Bn)
15.2. Japan Global Bioanalytical Testing Services Market
15.2.1. Japan Bioanalytical Testing Services Market Size and Forecast, 2023-2034 (Revenue USD Bn)
15.2.2. Japan Bioanalytical Testing Services Market Size and Forecast, By Molecule, 2023-2034 (Revenue USD Bn)
15.2.3. Japan Bioanalytical Testing Services Market Size and Forecast, By Workflow, 2023-2034 (Revenue USD Bn)
15.2.4. Japan Bioanalytical Testing Services Market Size and Forecast, By Test, 2023-2034 (Revenue USD Bn)
15.2.5. Japan Bioanalytical Testing Services Market Size and Forecast, By Therapeutic Area, 2023-2034 (Revenue USD Bn)
15.2.6. Japan Bioanalytical Testing Services Market Size and Forecast, By End-user, 2023-2034 (Revenue USD Bn)
15.3. China Global Bioanalytical Testing Services Market
15.3.1. China Bioanalytical Testing Services Market Size and Forecast, 2023-2034 (Revenue USD Bn)
15.3.2. China Bioanalytical Testing Services Market Size and Forecast, By Molecule, 2023-2034 (Revenue USD Bn)
15.3.3. China Bioanalytical Testing Services Market Size and Forecast, By Workflow, 2023-2034 (Revenue USD Bn)
15.3.4. China Bioanalytical Testing Services Market Size and Forecast, By Test, 2023-2034 (Revenue USD Bn)
15.3.5. China Bioanalytical Testing Services Market Size and Forecast, By Therapeutic Area, 2023-2034 (Revenue USD Bn)
15.3.6. China Bioanalytical Testing Services Market Size and Forecast, By End-user, 2023-2034 (Revenue USD Bn)
15.4. India Global Bioanalytical Testing Services Market
15.4.1. India Bioanalytical Testing Services Market Size and Forecast, 2023-2034 (Revenue USD Bn)
15.4.2. India Bioanalytical Testing Services Market Size and Forecast, By Molecule, 2023-2034 (Revenue USD Bn)
15.4.3. India Bioanalytical Testing Services Market Size and Forecast, By Workflow, 2023-2034 (Revenue USD Bn)
15.4.4. India Bioanalytical Testing Services Market Size and Forecast, By Test, 2023-2034 (Revenue USD Bn)
15.4.5. India Bioanalytical Testing Services Market Size and Forecast, By Therapeutic Area, 2023-2034 (Revenue USD Bn)
15.4.6. India Bioanalytical Testing Services Market Size and Forecast, By End-user, 2023-2034 (Revenue USD Bn)
15.5. South Korea Global Bioanalytical Testing Services Market
15.5.1. South Korea Bioanalytical Testing Services Market Size and Forecast, 2023-2034 (Revenue USD Bn)
15.5.2. South Korea Bioanalytical Testing Services Market Size and Forecast, By Molecule, 2023-2034 (Revenue USD Bn)
15.5.3. South Korea Bioanalytical Testing Services Market Size and Forecast, By Workflow, 2023-2034 (Revenue USD Bn)
15.5.4. South Korea Bioanalytical Testing Services Market Size and Forecast, By Test, 2023-2034 (Revenue USD Bn)
15.5.5. South Korea Bioanalytical Testing Services Market Size and Forecast, By Therapeutic Area, 2023-2034 (Revenue USD Bn)
15.5.6. South Korea Bioanalytical Testing Services Market Size and Forecast, By End-user, 2023-2034 (Revenue USD Bn)
15.6. Australia Global Bioanalytical Testing Services Market
15.6.1. Australia Bioanalytical Testing Services Market Size and Forecast, 2023-2034 (Revenue USD Bn)
15.6.2. Australia Bioanalytical Testing Services Market Size and Forecast, By Molecule, 2023-2034 (Revenue USD Bn)
15.6.3. Australia Bioanalytical Testing Services Market Size and Forecast, By Workflow, 2023-2034 (Revenue USD Bn)
15.6.4. Australia Bioanalytical Testing Services Market Size and Forecast, By Test, 2023-2034 (Revenue USD Bn)
15.6.5. Australia Bioanalytical Testing Services Market Size and Forecast, By Therapeutic Area, 2023-2034 (Revenue USD Bn)
15.6.6. Australia Bioanalytical Testing Services Market Size and Forecast, By End-user, 2023-2034 (Revenue USD Bn)
15.7. Rest of Asia Pacific Global Bioanalytical Testing Services Market
15.7.1. Rest of Asia Pacific Bioanalytical Testing Services Market Size and Forecast, 2023-2034 (Revenue USD Bn)
15.7.2. Rest of Asia Pacific Bioanalytical Testing Services Market Size and Forecast, By Molecule, 2023-2034 (Revenue USD Bn)
15.7.3. Rest of Asia Pacific Bioanalytical Testing Services Market Size and Forecast, By Workflow, 2023-2034 (Revenue USD Bn)
15.7.4. Rest of Asia Pacific Bioanalytical Testing Services Market Size and Forecast, By Test, 2023-2034 (Revenue USD Bn)
15.7.5. Rest of Asia Pacific Bioanalytical Testing Services Market Size and Forecast, By Therapeutic Area, 2023-2034 (Revenue USD Bn)
15.7.6. Rest of Asia Pacific Bioanalytical Testing Services Market Size and Forecast, By End-user, 2023-2034 (Revenue USD Bn)
16. Latin America Global Bioanalytical Testing Services Market
16.1. Latin America Global Bioanalytical Testing Services Market
16.1.1. Latin America Bioanalytical Testing Services Market Size and Forecast, 2023-2034 (Revenue USD Bn)
16.1.2. Latin America Bioanalytical Testing Services Market Size and Forecast, By Country, 2023-2034 (Revenue USD Bn)
16.1.3. Latin America Bioanalytical Testing Services Market Size and Forecast, By Molecule, 2023-2034 (Revenue USD Bn)
16.1.4. Latin America Bioanalytical Testing Services Market Size and Forecast, By Workflow, 2023-2034 (Revenue USD Bn)
16.1.5. Latin America Bioanalytical Testing Services Market Size and Forecast, By Test, 2023-2034 (Revenue USD Bn)
16.1.6. Latin America Bioanalytical Testing Services Market Size and Forecast, By Therapeutic Area, 2023-2034 (Revenue USD Bn)
16.1.7. Latin America Bioanalytical Testing Services Market Size and Forecast, By End-user, 2023-2034 (Revenue USD Bn)
16.2. Brazil Global Bioanalytical Testing Services Market
16.2.1. Brazil Bioanalytical Testing Services Market Size and Forecast, 2023-2034 (Revenue USD Bn)
16.2.2. Brazil Bioanalytical Testing Services Market Size and Forecast, By Molecule, 2023-2034 (Revenue USD Bn)
16.2.3. Brazil Bioanalytical Testing Services Market Size and Forecast, By Workflow, 2023-2034 (Revenue USD Bn)
16.2.4. Brazil Bioanalytical Testing Services Market Size and Forecast, By Test, 2023-2034 (Revenue USD Bn)
16.2.5. Brazil Bioanalytical Testing Services Market Size and Forecast, By Therapeutic Area, 2023-2034 (Revenue USD Bn)
16.2.6. Brazil Bioanalytical Testing Services Market Size and Forecast, By End-user, 2023-2034 (Revenue USD Bn)
16.3. Mexico Global Bioanalytical Testing Services Market
16.3.1. Mexico Bioanalytical Testing Services Market Size and Forecast, 2023-2034 (Revenue USD Bn)
16.3.2. Mexico Bioanalytical Testing Services Market Size and Forecast, By Molecule, 2023-2034 (Revenue USD Bn)
16.3.3. Mexico Bioanalytical Testing Services Market Size and Forecast, By Workflow, 2023-2034 (Revenue USD Bn)
16.3.4. Mexico Bioanalytical Testing Services Market Size and Forecast, By Test, 2023-2034 (Revenue USD Bn)
16.3.5. Mexico Bioanalytical Testing Services Market Size and Forecast, By Therapeutic Area, 2023-2034 (Revenue USD Bn)
16.3.6. Mexico Bioanalytical Testing Services Market Size and Forecast, By End-user, 2023-2034 (Revenue USD Bn)
16.4. Argentina Global Bioanalytical Testing Services Market
16.4.1. Argentina Bioanalytical Testing Services Market Size and Forecast, 2023-2034 (Revenue USD Bn)
16.4.2. Argentina Bioanalytical Testing Services Market Size and Forecast, By Molecule, 2023-2034 (Revenue USD Bn)
16.4.3. Argentina Bioanalytical Testing Services Market Size and Forecast, By Workflow, 2023-2034 (Revenue USD Bn)
16.4.4. Argentina Bioanalytical Testing Services Market Size and Forecast, By Test, 2023-2034 (Revenue USD Bn)
16.4.5. Argentina Bioanalytical Testing Services Market Size and Forecast, By Therapeutic Area, 2023-2034 (Revenue USD Bn)
16.4.6. Argentina Bioanalytical Testing Services Market Size and Forecast, By End-user, 2023-2034 (Revenue USD Bn)
16.5. Rest of Latin America Global Bioanalytical Testing Services Market
16.5.1. Rest of Latin America Bioanalytical Testing Services Market Size and Forecast, 2023-2034 (Revenue USD Bn)
16.5.2. Rest of Latin America Bioanalytical Testing Services Market Size and Forecast, By Molecule, 2023-2034 (Revenue USD Bn)
16.5.3. Rest of Latin America Bioanalytical Testing Services Market Size and Forecast, By Workflow, 2023-2034 (Revenue USD Bn)
16.5.4. Rest of Latin America Bioanalytical Testing Services Market Size and Forecast, By Test, 2023-2034 (Revenue USD Bn)
16.5.5. Rest of Latin America Bioanalytical Testing Services Market Size and Forecast, By Therapeutic Area, 2023-2034 (Revenue USD Bn)
16.5.6. Rest of Latin America Bioanalytical Testing Services Market Size and Forecast, By End-user, 2023-2034 (Revenue USD Bn)
17. MEA Global Bioanalytical Testing Services Market
17.1. MEA Global Bioanalytical Testing Services Market
17.1.1. MEA Bioanalytical Testing Services Market Size and Forecast, 2023-2034 (Revenue USD Bn)
17.1.2. MEA Bioanalytical Testing Services Market Size and Forecast, By Country, 2023-2034 (Revenue USD Bn)
17.1.3. MEA Bioanalytical Testing Services Market Size and Forecast, By Molecule, 2023-2034 (Revenue USD Bn)
17.1.4. MEA Bioanalytical Testing Services Market Size and Forecast, By Workflow, 2023-2034 (Revenue USD Bn)
17.1.5. MEA Bioanalytical Testing Services Market Size and Forecast, By Test, 2023-2034 (Revenue USD Bn)
17.1.6. MEA Bioanalytical Testing Services Market Size and Forecast, By Therapeutic Area, 2023-2034 (Revenue USD Bn)
17.1.7. MEA Bioanalytical Testing Services Market Size and Forecast, By End-user, 2023-2034 (Revenue USD Bn)
17.2. GCC Global Bioanalytical Testing Services Market
17.2.1. GCC Bioanalytical Testing Services Market Size and Forecast, 2023-2034 (Revenue USD Bn)
17.2.2. GCC Bioanalytical Testing Services Market Size and Forecast, By Molecule, 2023-2034 (Revenue USD Bn)
17.2.3. GCC Bioanalytical Testing Services Market Size and Forecast, By Workflow, 2023-2034 (Revenue USD Bn)
17.2.4. GCC Bioanalytical Testing Services Market Size and Forecast, By Test, 2023-2034 (Revenue USD Bn)
17.2.5. GCC Bioanalytical Testing Services Market Size and Forecast, By Therapeutic Area, 2023-2034 (Revenue USD Bn)
17.2.6. GCC Bioanalytical Testing Services Market Size and Forecast, By End-user, 2023-2034 (Revenue USD Bn)
17.3. South Africa Global Bioanalytical Testing Services Market
17.3.1. South Africa Bioanalytical Testing Services Market Size and Forecast, 2023-2034 (Revenue USD Bn)
17.3.2. South Africa Bioanalytical Testing Services Market Size and Forecast, By Molecule, 2023-2034 (Revenue USD Bn)
17.3.3. South Africa Bioanalytical Testing Services Market Size and Forecast, By Workflow, 2023-2034 (Revenue USD Bn)
17.3.4. South Africa Bioanalytical Testing Services Market Size and Forecast, By Test, 2023-2034 (Revenue USD Bn)
17.3.5. South Africa Bioanalytical Testing Services Market Size and Forecast, By Therapeutic Area, 2023-2034 (Revenue USD Bn)
17.3.6. South Africa Bioanalytical Testing Services Market Size and Forecast, By End-user, 2023-2034 (Revenue USD Bn)
17.4. Rest of MEA Global Bioanalytical Testing Services Market
17.4.1. Rest of MEA Bioanalytical Testing Services Market Size and Forecast, 2023-2034 (Revenue USD Bn)
17.4.2. Rest of MEA Bioanalytical Testing Services Market Size and Forecast, By Molecule, 2023-2034 (Revenue USD Bn)
17.4.3. Rest of MEA Bioanalytical Testing Services Market Size and Forecast, By Workflow, 2023-2034 (Revenue USD Bn)
17.4.4. Rest of MEA Bioanalytical Testing Services Market Size and Forecast, By Test, 2023-2034 (Revenue USD Bn)
17.4.5. Rest of MEA Bioanalytical Testing Services Market Size and Forecast, By Therapeutic Area, 2023-2034 (Revenue USD Bn)
17.4.6. Rest of MEA Bioanalytical Testing Services Market Size and Forecast, By End-user, 2023-2034 (Revenue USD Bn)
18. Competitor Analysis
18.1. Company Market Share Analysis, 2023
18.2. Major Recent Developments
19. Company Profiles
19.1. Pace Analytical Services LLC
19.2. SGS SA
19.3. Intertek Group Plc
19.4. PPD, Inc.
19.5. ICON Plc
19.6. Syneos Health
19.7. IQVIA
19.8. Covance, Inc.
19.9. Toxikon
19.10. Charles River Laboratories International
19.11. Other Prominent Players