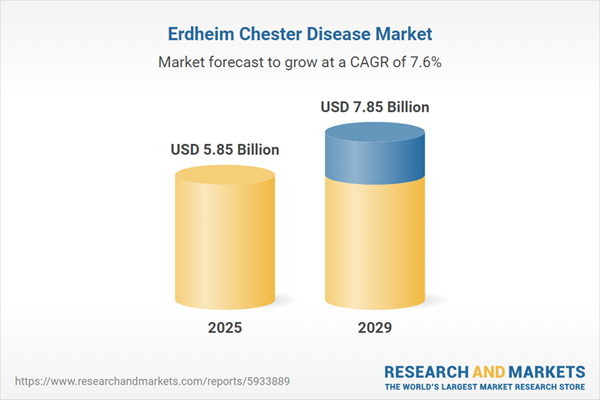
The erdheim chester disease market size has grown strongly in recent years. It will grow from $5.4 billion in 2024 to $5.85 billion in 2025 at a compound annual growth rate (CAGR) of 8.3%. The growth in the historic period can be attributed to increased disease awareness, collaborative research initiatives, rare disease recognition, global patient advocacy, regulatory support for orphan drugs.
The erdheim chester disease market size is expected to see strong growth in the next few years. It will grow to $7.85 billion in 2029 at a compound annual growth rate (CAGR) of 7.6%. The growth in the forecast period can be attributed to precision medicine approaches, advancements in imaging techniques, expansion of clinical trial participation, increased funding for rare diseases, emergence of novel therapeutic targets. Major trends in the forecast period include patient-centric drug development, international collaborations, advancements in diagnostic technologies, research on genetic and molecular basis, immunotherapeutic advancements.
The rising number of clinical trials is anticipated to drive the growth of the Erdheim-Chester disease market in the future. Clinical trials are research studies designed to assess the safety, effectiveness, and potential benefits of new medical treatments, interventions, drugs, devices, or procedures. They play a crucial role in the development of innovative therapies for Erdheim-Chester disease. These studies examine the pathophysiological and clinical aspects of the condition, collect patient data to enhance understanding, and evaluate the safety and efficacy of new treatments. For instance, as reported by Xtalks, a digital medical company based in Canada, there were 452,604 registered clinical trials globally on ClinicalTrials.gov as of May 2023. Out of these, 64,838 trials were actively recruiting participants, marking a significant increase from the over 365,000 registered trials noted in early 2021. Thus, the growing number of clinical trials is fueling the expansion of the Erdheim-Chester disease market.
The increasing healthcare expenditure is projected to enhance the growth of the Erdheim-Chester disease market in the coming years. Healthcare expenditure refers to the total amount spent by individuals on healthcare services and products. A rise in healthcare spending for Erdheim-Chester disease can lead to improved care, better treatments, and a more favorable outlook for patients, while also promoting advancements in medical science and benefiting society as a whole. For example, according to statistics from Cross River Therapy, a US-based provider of ABA therapy services, the U.S. pharmaceutical industry generated $550 billion in revenue, with Americans spending $576.9 billion on medications in 2021, and projected spending is expected to rise to between $605 billion and $635 billion by 2025. Therefore, the increase in healthcare expenditure is propelling the growth of the Erdheim-Chester disease market moving forward.
Key players in the Erdheim-Chester disease market are at the forefront of innovation, developing groundbreaking products such as the combination of dabrafenib (Tafinlar) with trametinib (Mekinist). This strategic approach, coupled with obtaining regulatory approvals, aims to cater to larger customer bases, drive increased sales, and boost overall revenue. An illustrative example is the approval of dabrafenib by the Food and Drug Administration (FDA) in March 2023. Dabrafenib, a BRAF inhibitor, has proven efficacy in treating Erdheim-Chester disease (ECD), a rare form of non-Langerhans cell histiocytosis. Notably, dabrafenib has demonstrated effectiveness in ECD patients, particularly those with lesions positive for the BRAF V600E mutation. Pediatric ECD patients, especially those with significant cerebral lesions, have exhibited positive responses to dabrafenib treatment.
Key companies in the Erdheim-Chester disease market are concentrating on the development of innovative products, such as targeted cancer therapies, to offer viable treatment options for adult patients with histiocytic neoplasms, thereby addressing an unmet need in the management of these rare cancers. Targeted cancer therapy refers to treatments that specifically attack the unique characteristics of cancer cells, such as genetic mutations or particular proteins, to inhibit their growth and survival while minimizing harm to normal cells. For example, in November 2022, the US Food and Drug Administration (FDA), a federal agency in the United States, granted approval for cobimetinib (Cotellic) for the treatment of adult patients with histiocytic neoplasms, which include Erdheim-Chester disease, Rosai-Dorfman disease, and Langerhans cell histiocytosis. The trials for this study included patients with histologically confirmed histiocytic neoplasms of any mutational status.
Major companies operating in the erdheim chester disease market report are Pfizer Inc., Johnson & Johnson, Roche Holding AG, AbbVie Inc., Bayer AG, Novartis AG, Sanofi S.A., Bristol-Myers Squibb Company, AstraZeneca PLC, GSK PLC, Eli Lilly and Company, Amgen Inc., Teva Pharmaceutical Industries Ltd., Regeneron Pharmaceuticals Inc., Mylan N.V., Astellas Pharma Inc., Vertex Pharmaceuticals, Sun Pharmaceutical Industries Limited, Horizon Therapeutics PLC, Hikma Pharmaceuticals PLC, BioMarin Pharmaceutical Inc., Cipla Inc., Jubilant Pharmova Limited, Labcorp, Orchard Therapeutics PLC, Cyclerion Therapeutics Inc.
North America was the largest region in the Erdheim-Chester disease market in 2024. Asia-Pacific is expected to be the fastest-growing region in the forecast period. The regions covered in the erdheim chester disease market report are Asia-Pacific, Western Europe, Eastern Europe, North America, South America, Middle East, Africa. The countries covered in the erdheim chester disease market report are Australia, Brazil, China, France, Germany, India, Indonesia, Japan, Russia, South Korea, UK, USA, Canada, Italy, Spain.
The erdheim-chester disease market includes revenues earned by entities by providing services such as imaging services, diagnostic services, pathology services, chemotherapy, targeted therapy, supportive care, clinical trials, and others. The market value includes the value of related goods sold by the service provider or included within the service offering. The Erdheim-Chester disease market also includes sales of vemurafenib, dabrafenib, interferon-alpha, anakinra, cladribine, cytarabine, and bisphosphonates. Values in this market are ‘factory gate’ values, that is the value of goods sold by the manufacturers or creators of the goods, whether to other entities (including downstream manufacturers, wholesalers, distributors, and retailers) or directly to end customers. The value of goods in this market includes related services sold by the creators of the goods.
Erdheim-Chester disease (ECD) is an extremely rare form of non-Langerhans cell histiocytosis, characterized by the abnormal accumulation and proliferation of histiocytes - immune system cells that play a role in inflammation and immune response. ECD specifically involves the excessive production and accumulation of histiocytes, resulting in the formation of granulomatous lesions in various organs.
The primary treatments for Erdheim-Chester disease include chemotherapy, immunotherapy, radiation therapy, and other therapeutic approaches. Chemotherapy is a medical intervention that employs potent drugs to target and eliminate rapidly dividing cells, such as cancer cells. It can be administered through various routes, including oral and parenteral methods. These treatments are distributed through different channels, such as hospital pharmacies, online pharmacies, and retail pharmacies, catering to diverse end users such as hospitals, homecare settings, specialty clinics, and others.
The erdheim chester disease market research report is one of a series of new reports that provides erdheim chester disease market statistics, including erdheim chester disease industry global market size, regional shares, competitors with an erdheim chester disease market share, detailed erdheim chester disease market segments, market trends and opportunities, and any further data you may need to thrive in the erdheim chester disease industry. This erdheim chester disease market research report delivers a complete perspective of everything you need, with an in-depth analysis of the current and future scenario of the industry.
The market value is defined as the revenues that enterprises gain from the sale of goods and/or services within the specified market and geography through sales, grants, or donations in terms of the currency (in USD, unless otherwise specified).
The revenues for a specified geography are consumption values that are revenues generated by organizations in the specified geography within the market, irrespective of where they are produced. It does not include revenues from resales along the supply chain, either further along the supply chain or as part of other products.
This product will be delivered within 3-5 business days.
Table of Contents
Executive Summary
Erdheim Chester Disease Global Market Report 2025 provides strategists, marketers and senior management with the critical information they need to assess the market.This report focuses on erdheim chester disease market which is experiencing strong growth. The report gives a guide to the trends which will be shaping the market over the next ten years and beyond.
Reasons to Purchase:
- Gain a truly global perspective with the most comprehensive report available on this market covering 15 geographies.
- Assess the impact of key macro factors such as conflict, pandemic and recovery, inflation and interest rate environment and the 2nd Trump presidency.
- Create regional and country strategies on the basis of local data and analysis.
- Identify growth segments for investment.
- Outperform competitors using forecast data and the drivers and trends shaping the market.
- Understand customers based on the latest market shares.
- Benchmark performance against key competitors.
- Suitable for supporting your internal and external presentations with reliable high quality data and analysis
- Report will be updated with the latest data and delivered to you along with an Excel data sheet for easy data extraction and analysis.
- All data from the report will also be delivered in an excel dashboard format.
Description
Where is the largest and fastest growing market for erdheim chester disease? How does the market relate to the overall economy, demography and other similar markets? What forces will shape the market going forward? The erdheim chester disease market global report answers all these questions and many more.The report covers market characteristics, size and growth, segmentation, regional and country breakdowns, competitive landscape, market shares, trends and strategies for this market. It traces the market’s historic and forecast market growth by geography.
- The market characteristics section of the report defines and explains the market.
- The market size section gives the market size ($b) covering both the historic growth of the market, and forecasting its development.
- The forecasts are made after considering the major factors currently impacting the market. These include:
- The forecasts are made after considering the major factors currently impacting the market. These include the Russia-Ukraine war, rising inflation, higher interest rates, and the legacy of the COVID-19 pandemic.
- Market segmentations break down the market into sub markets.
- The regional and country breakdowns section gives an analysis of the market in each geography and the size of the market by geography and compares their historic and forecast growth. It covers the growth trajectory of COVID-19 for all regions, key developed countries and major emerging markets.
- The competitive landscape chapter gives a description of the competitive nature of the market, market shares, and a description of the leading companies. Key financial deals which have shaped the market in recent years are identified.
- The trends and strategies section analyses the shape of the market as it emerges from the crisis and suggests how companies can grow as the market recovers.
Scope
Markets Covered:
1) By Treatment: Chemotherapy; Immunotherapy; Radiation Therapy; Other Treatments2) By Route Of Administration: Oral; Parenteral; Other Route Of Administration
3) By Distribution Channel: Hospital Pharmacy; Online Pharmacy; Retail Pharmacy
4) By End User: Hospitals; Homecare; Specialty Clinics; Other End User
Subsegments:
1) By Chemotherapy: Traditional Chemotherapy Agents; Targeted Chemotherapy Regimens2) By Immunotherapy: Checkpoint Inhibitors; Monoclonal Antibodies; Interferon Therapy
3) By Radiation Therapy: Palliative Radiation Therapy; Stereotactic Radiosurgery
4) By Other Treatments: Targeted Therapy; Supportive Care And Symptomatic Treatment; Clinical Trials For Novel Therapies
Key Companies Mentioned: Pfizer Inc.; Johnson & Johnson; Roche Holding AG; AbbVie Inc.; Bayer AG
Countries: Australia; Brazil; China; France; Germany; India; Indonesia; Japan; Russia; South Korea; UK; USA; Canada; Italy; Spain
Regions: Asia-Pacific; Western Europe; Eastern Europe; North America; South America; Middle East; Africa
Time Series: Five years historic and ten years forecast.
Data: Ratios of market size and growth to related markets, GDP proportions, expenditure per capita.
Data Segmentation: Country and regional historic and forecast data, market share of competitors, market segments.
Sourcing and Referencing: Data and analysis throughout the report is sourced using end notes.
Delivery Format: PDF, Word and Excel Data Dashboard.
Companies Mentioned
Some of the major companies featured in this Erdheim Chester Disease market report include:- Pfizer Inc.
- Johnson & Johnson
- Roche Holding AG
- AbbVie Inc.
- Bayer AG
- Novartis AG
- Sanofi S.A.
- Bristol-Myers Squibb Company
- AstraZeneca plc
- GSK plc
- Eli Lilly and Company
- Amgen Inc.
- Teva Pharmaceutical Industries Ltd.
- Regeneron Pharmaceuticals Inc.
- Mylan N.V.
- Astellas Pharma Inc.
- Vertex Pharmaceuticals
- Sun Pharmaceutical Industries Limited
- Horizon Therapeutics plc
- Hikma Pharmaceuticals plc
- BioMarin Pharmaceutical Inc.
- Cipla Inc.
- Jubilant Pharmova Limited
- Labcorp
- Orchard Therapeutics plc
- Cyclerion Therapeutics Inc
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 200 |
| Published | March 2025 |
| Forecast Period | 2025 - 2029 |
| Estimated Market Value ( USD | $ 5.85 Billion |
| Forecasted Market Value ( USD | $ 7.85 Billion |
| Compound Annual Growth Rate | 7.6% |
| Regions Covered | Global |
| No. of Companies Mentioned | 27 |