The market in Asia Pacific region is being driven by factors like expanding medical tourism, inexpensive treatment options, technological development, and increasing healthcare reimbursements. Thus, the APAC region generated $1,009.7 million revenue in the market in 2022. Furthermore, the demand for hernia repair devices is rising in Asia, particularly Japan and China, due to the region's large patient population. Many undiagnosed and untreated cases are anticipated to fuel the market expansion in this region, which is expected to experience robust growth. Some of the factors impacting the market are increasing prevalence of hernias, improved fixation techniques, and complications leading to revision surgeries.
The growing prevalence of hernias, including inguinal, incisional, ventral, umbilical, and femoral hernias, is a primary driver of the market. Factors such as an aging population, obesity rates, and lifestyle choices contribute to the rise in hernia cases. As per a report by the National Library of Medicine updated in 2023, there were 32.53 million prevalent cases and 13.02 million incident cases of inguinal, femoral, and abdominal hernias globally in 2019, representing increases of 36.00% and 63.67 %, respectively. Medical conditions like chronic obstructive pulmonary disease, constipation, and other factors that cause chronic coughing or straining can lead to an increased risk of hernias. Additionally, Innovations in mesh fixation techniques, including self-gripping meshes, have simplified the surgical process, and reduced the need for additional fixation devices, making hernia repair procedures more efficient. Improved fixation methods lead to shorter operating times. This is particularly valuable for surgeons and healthcare facilities as it allows for more efficient resource utilization and increased surgeries performed in each time frame. Many advanced fixation techniques are well-suited for minimally invasive surgical procedures, such as laparoscopy and robotic-assisted surgery. The growing adoption of fixation techniques improves the overall quality of hernia repair surgeries. This, in turn, contributes to the sustained growth of the market.
However, some patients experience complications related to hernia mesh devices, such as infections, chronic pain, or mesh migration. These complications can lead to revision surgeries, impacting patient satisfaction and increasing healthcare costs. Complications from hernia mesh procedures, such as infections, chronic pain, or mesh migration, can erode patient confidence and trust in these devices. Patients who experience complications hesitate to undergo further hernia repair surgeries, affecting the market's growth. Complications from hernia mesh devices have led to lawsuits and legal actions against manufacturers. In addition, manufacturers face increased regulatory challenges. Thus, difficulties and the need for revision surgeries challenge the market.
The pandemic caused supply chain disruptions for medical devices, such as hernia mesh products. Lockdowns, travel restrictions, and increased demand for personal protective equipment (PPE) affected the production and distribution of hernia mesh devices. This resulted in manufacturing and delivery delays, affecting healthcare facilities' ability to perform hernia surgeries. Many healthcare systems and governments around the world postponed or canceled elective surgeries to prioritize resources for COVID-19 patients. Hernia repair surgeries, especially those considered non-urgent, were deferred. This significantly impacted the demand for hernia mesh devices, as fewer surgeries were being performed.
Hernia Type Outlook
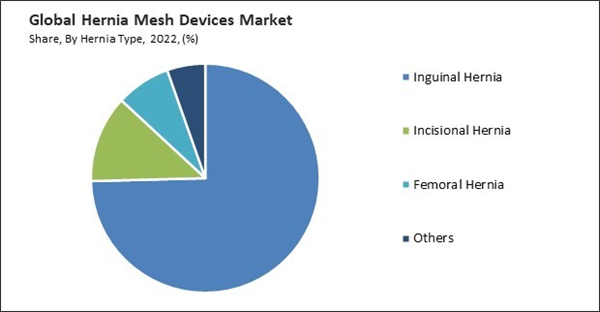
By hernia type, the market is categorized into inguinal hernia, incisional hernia, femoral hernia, and others. In 2022, the inguinal hernia segment held the highest revenue share in the market. The rising prevalence of inguinal hernias, influenced by aging populations and lifestyle-related risk factors, fuels the demand for hernia mesh devices. As inguinal hernias become more common, healthcare providers are more likely to utilize hernia mesh devices in their treatment protocols. Hernia mesh devices effectively reduce the risk of hernia recurrence in inguinal hernia repair. Using mesh offers better outcomes and patient satisfaction, encouraging surgeons and healthcare facilities to adopt these devices.Type Outlook
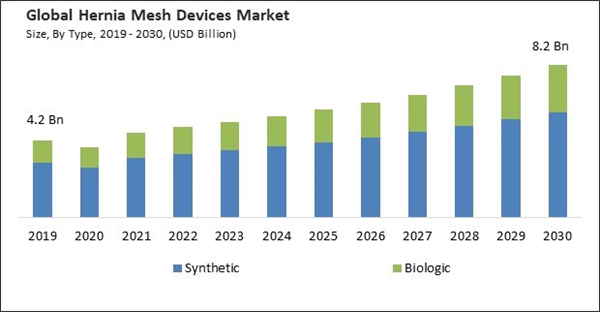
Based on type, the market is bifurcated into biologic and synthetic. In 2022, the biologic mesh segment witnessed a substantial revenue share in the market. Biologic meshes promote the natural tissue repair and remodeling process. Over time, these meshes are gradually absorbed by the patient's body and replaced with their tissue. This feature is especially beneficial for patients who require long-term structural support but may be at risk of synthetic mesh-related complications. The safety profile of biologic meshes is superior for specific patient populations, such as those with infections, contaminated wounds, or a history of mesh-related complications. Biologic meshes are less prone to complications like infection, erosion, or adhesion formation.Regional Outlook
Region-wise, the market is analysed across North America, Europe, Asia Pacific, and LAMEA. In 2022, the North America region led the market by generating the highest revenue share. The market in North America includes a wide range of hernia mesh devices, including synthetic meshes, biologic meshes, and composite meshes. Sedentary lifestyles, a growing elderly population, and a high risk of hernia recurrence drive North America's market expansion.The market research report covers the analysis of key stake holders of the market. Key companies profiled in the report include Becton Dickinson and Company, Johnson & Johnson, W. L. Gore & Associates, Inc., Atrium Medical Corporation (Getinge AB), B. Braun Melsungen AG, Baxter International, Inc., Cook Medical, Inc. (Cook Group), Medtronic PLC, Integra LifeSciences Holdings Corporation, and Herniamesh S.r.l.
Scope of the Study
Market Segments Covered in the Report:
By Type (Volume, thousand units, USD Million, 2019-2030)- Synthetic
- Biologic
- Inguinal Hernia
- Incisional Hernia
- Femoral Hernia
- Others
- North America
- US
- Canada
- Mexico
- Rest of North America- Europe
- Germany
- UK
- France
- Russia
- Spain
- Italy
- Rest of Europe- Asia Pacific
- China
- Japan
- India
- South Korea
- Singapore
- Malaysia
- Rest of Asia Pacific- LAMEA
- Brazil
- Argentina
- UAE
- Saudi Arabia
- South Africa
- Nigeria
- Rest of LAMEA
Key Market Players
List of Companies Profiled in the Report:
- Becton Dickinson and Company
- Johnson & Johnson
- W.L. Gore & Associates, Inc.
- Atrium Medical Corporation (Getinge AB)
- B.Braun Melsungen AG
- Baxter International, Inc.
- Cook Medical, Inc. (Cook Group)
- Medtronic PLC
- Integra LifeSciences Holdings Corporation
- Herniamesh S.r.l.
Unique Offerings
- Exhaustive coverage
- The highest number of Market tables and figures
- Subscription-based model available
- Guaranteed best price
- Assured post sales research support with 10% customization free
Table of Contents
Companies Mentioned
- Becton Dickinson and Company
- Johnson & Johnson
- W. L. Gore & Associates, Inc.
- Atrium Medical Corporation (Getinge AB)
- B. Braun Melsungen AG
- Baxter International, Inc.
- Cook Medical, Inc. (Cook Group)
- Medtronic PLC
- Integra LifeSciences Holdings Corporation
- Herniamesh S.r.l.