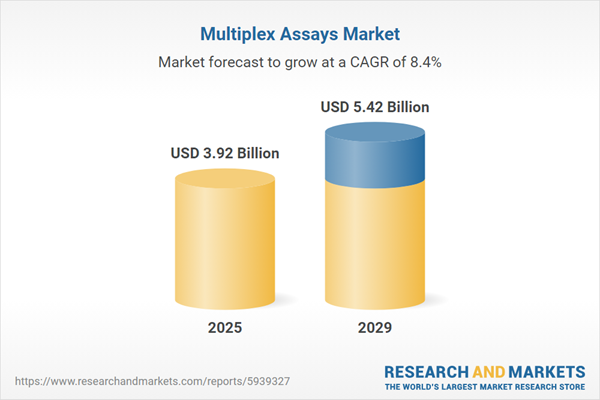
The multiplex assays market size is expected to see strong growth in the next few years. It will grow to $5.42 billion in 2029 at a compound annual growth rate (CAGR) of 8.4%. The growth in the forecast period can be attributed to the rise in funding, government initiatives for genetic and microbiological research, aging population, increasing prevalence of chronic diseases and a rapid rise in various bacterial and viral epidemics. Major trends in the forecast period include the rise in funding, government initiatives for genetic and microbiological research, the aging population, the increasing prevalence of chronic diseases, and a rapid rise in various bacterial and viral epidemics.
The increasing incidence of chronic diseases is fueling growth in the global multiplex assay market. Multiplex assays are widely used in clinical trials to assess disease efficacy and to detect antibodies related to various conditions. For instance, in September 2023, a report by the World Health Organization (WHO) highlighted that chronic diseases, also known as non-communicable diseases (NCDs), are responsible for 74% of global deaths, amounting to 41 million fatalities annually. Consequently, the growing prevalence of chronic diseases is expected to drive demand in the global multiplex assay market.
The market for multiplex assays is expected to be further driven by increased funding from governments and private organizations. Significant investments are being made to develop multiplex assays, especially amid the challenges posed by the COVID-19 pandemic. The surge in COVID-19 testing has led to consolidation and merger activities. For instance, in May 2024, Autonomous Medical Devices Incorporated (AMDI), a U.S.-based biotechnology company, received a $5.2 million grant from the National Institutes of Health (NIH) to finalize its rapid point-of-care (POC) viral testing system. This system employs polymerase chain reaction (PCR), a highly regarded method for amplifying specific DNA sequences, enabling accurate detection of viral genetic material. With its capability to identify even minimal amounts of viral RNA or DNA, the system offers high sensitivity and specificity, making it a valuable tool for diagnosing infections at their early stages.
Companies in the market are actively launching innovative tests based on multiplex assays to fortify their market positions. In May 2022, QuantuMDx Group Limited introduced the Q-POC SARS-CoV-2, Flu A/B, and RSV Assay, a multiplex panel designed to detect SARS-CoV-2, Influenza A, Influenza B, and RSV at the point of need, further showcasing the market's evolution.
Companies in the multiplex assay market are prioritizing new product launches to enhance their market presence. For example, in January 2024, Bio-Rad Laboratories Inc., a U.S.-based company specializing in life science research and clinical diagnostic products, introduced the ddPLEX ESR1 Mutation Detection Kit. This kit is the first ultrasensitive multiplexed digital PCR assay designed specifically for detecting breast cancer mutations in clinical research. It allows for the simultaneous detection and quantification of seven relevant ESR1 mutations in a single well, simplifying the testing process. With an analytical sensitivity of 0.01% variant allele fraction (VAF), it can detect low-frequency mutations, which are crucial in cancer diagnostics. Additionally, the kit is compatible with circulating tumor DNA (ctDNA) from plasma and DNA from formalin-fixed paraffin-embedded (FFPE) tissue samples, offering flexibility in sample types for testing.
In May 2022, CellCarta, a Canada-based precision laboratory services company, acquired Precision Assays for an undisclosed amount. This acquisition aims to expand CellCarta’s precision medicine laboratory services by obtaining commercial rights to antibody panels and assays developed by Precision Assays. Through this acquisition, CellCarta enhances its capabilities in next-generation targeted proteomics testing solutions, especially benefiting its pharmaceutical and biotech clients. Precision Assays, a U.S.-based developer, specializes in high-end multiplex quantitative immuno-MRM mass spectrometry-based assays, adding advanced analytical capabilities to CellCarta’s service offerings.
Major companies operating in the multiplex assays market include Seegene Inc, DiaSorin SpA, Thermo Fisher Scientific Inc, Bio-Rad Laboratories Inc, Abcam Plc, PerkinElmer Inc, Hologic Corporation, Meso Scale Diagnostics, Merck KGaA, Roche Diagnostics India Pvt Ltd, bioMérieux India, Becton Dickinson Private Limited, Danaher Corporation, Johnson & Johnson, Simens Healthcare, Beckman Coulter Inc. India, Ortho Clinical Diagnostics, Sysmex Corporation, Siemens Healthineers Ukraine, TestLine Clinical Diagnostics s.r.o., Cepheid, F. Hoffmann-La Roche Ltd., Hologic, Bayer AG, Cantel Medical Corporation, Meridian Bioscience, Faizyme Laboratories, Alere Inc., Bangs Laboratories Inc, The Merck Group, Takara Bio Inc, Qiagen, Spherotech Inc, Geneaid Biotech Ltd, Rockland Immunochemicals Inc, GenScript Biotech Corporation.
North America was the largest region in the multiplex assays market in 2024. Asia-Pacific is expected to be the fastest-growing region in the forecast period. The regions covered in the multiplex assays market report are Asia-Pacific, Western Europe, Eastern Europe, North America, South America, Middle East, Africa. The countries covered in the multiplex assays market report are Australia, Brazil, China, France, Germany, India, Indonesia, Japan, Russia, South Korea, UK, USA, Italy, Spain, Canada.
Multiplex assays are employed to amplify multiple targets in a polymerase chain reaction (PCR) trial, enabling the extraction of additional information from minuscule amounts of proteins or other analyses in a shorter time compared to traditional systems, similar to ELISA. These assays find application in pathogen identification, mutation analysis, RNA discovery, gene discovery analysis, relation analysis, forensic studies, and other scientific endeavors.
The primary types of multiplex assays comprise nucleic acid-based multiplex assays, protein-based multiplex assays, and others. Nucleic acid-based multiplex assays rely on nucleic acids to detect organism-specific DNA or RNA sequences. These tests are generally characterized by specificity and high sensitivity, providing rapid results. Various technologies underpin multiplex assays, including flow cytometry, luminescence, fluorescence detection, multiplex real-time PCR, and other related technologies. Multiplex assays serve diverse applications, including research and development, as well as clinical diagnostics. End-users encompass pharmaceutical and biotechnology companies, hospitals and research institutes, reference laboratories, and other entities involved in scientific research and diagnostics.
The multiplex assays market research report is one of a series of new reports that provides multiplex assays market statistics, including multiplex assays industry global market size, regional shares, competitors with multiplex assays market share, detailed multiplex assays market segments, market trends and opportunities, and any further data you may need to thrive in the multiplex assays industry. This multiplex assays market research report delivers a complete perspective of everything you need, with an in-depth analysis of the current and future scenario of the industry.
The multiplex assays market consists of sales of equipment with technologies that can detect multiple genes such as flow cytometry, fluorescence detection, luminescence, multiplex real-time PCR, and other technologies in multiplex assays. The market value includes the value of related goods sold by the service provider or included within the service offering. Only goods and services traded between entities or sold to end consumers are included.
The market value is defined as the revenues that enterprises gain from the sale of goods and/or services within the specified market and geography through sales, grants, or donations in terms of the currency (in USD unless otherwise specified).
The revenues for a specified geography are consumption values that are revenues generated by organizations in the specified geography within the market, irrespective of where they are produced. It does not include revenues from resales along the supply chain, either further along the supply chain or as part of other products.
This product will be delivered within 3-5 business days.
Table of Contents
Executive Summary
Multiplex Assays Global Market Report 2025 provides strategists, marketers and senior management with the critical information they need to assess the market.This report focuses on multiplex assays market which is experiencing strong growth. The report gives a guide to the trends which will be shaping the market over the next ten years and beyond.
Reasons to Purchase:
- Gain a truly global perspective with the most comprehensive report available on this market covering 15 geographies.
- Assess the impact of key macro factors such as conflict, pandemic and recovery, inflation and interest rate environment and the 2nd Trump presidency.
- Create regional and country strategies on the basis of local data and analysis.
- Identify growth segments for investment.
- Outperform competitors using forecast data and the drivers and trends shaping the market.
- Understand customers based on the latest market shares.
- Benchmark performance against key competitors.
- Suitable for supporting your internal and external presentations with reliable high quality data and analysis
- Report will be updated with the latest data and delivered to you along with an Excel data sheet for easy data extraction and analysis.
- All data from the report will also be delivered in an excel dashboard format.
Description
Where is the largest and fastest growing market for multiplex assays? How does the market relate to the overall economy, demography and other similar markets? What forces will shape the market going forward? The multiplex assays market global report answers all these questions and many more.The report covers market characteristics, size and growth, segmentation, regional and country breakdowns, competitive landscape, market shares, trends and strategies for this market. It traces the market’s historic and forecast market growth by geography.
- The market characteristics section of the report defines and explains the market.
- The market size section gives the market size ($b) covering both the historic growth of the market, and forecasting its development.
- The forecasts are made after considering the major factors currently impacting the market. These include:
- The forecasts are made after considering the major factors currently impacting the market. These include the Russia-Ukraine war, rising inflation, higher interest rates, and the legacy of the COVID-19 pandemic.
- Market segmentations break down the market into sub markets.
- The regional and country breakdowns section gives an analysis of the market in each geography and the size of the market by geography and compares their historic and forecast growth. It covers the growth trajectory of COVID-19 for all regions, key developed countries and major emerging markets.
- The competitive landscape chapter gives a description of the competitive nature of the market, market shares, and a description of the leading companies. Key financial deals which have shaped the market in recent years are identified.
- The trends and strategies section analyses the shape of the market as it emerges from the crisis and suggests how companies can grow as the market recovers.
Scope
Markets Covered:
1) By Type: Protein-Based Multiplex Assays, Nucleic Acid-Based Multiplex Assays, Other Types2) By Identification Technology: Flow Cytometry, Multiplex Real-Time PCR, Other Identification Technologies
3) By Detection Technology: Enzyme-Linked Immunosorbent Assay (ELISA), Luminescence, Fluorescence, Other Detection Technologies
4) By Application: Research and Development, Clinical Diagnostics
5) By End User: Pharmaceutical and Biotechnology Companies, Hospitals and Research Institutes, Reference Laboratories, Other End-Users
Subsegments:
1) By Protein-Based Multiplex Assays: ELISA (Enzyme-Linked Immunosorbent Assay); Luminex xMAP Technology; Western Blotting; Immunohistochemistry; Mass Spectrometry-Based Assays2) By Nucleic Acid-Based Multiplex Assays: PCR (Polymerase Chain Reaction); qPCR (Quantitative PCR); NGS (Next-Generation Sequencing); Microarray Analysis; RT-PCR (Reverse Transcription PCR)
3) By Other Types: Cell-Based Multiplex Assays; Bioanalytical Assays; Imaging-Based Assays; Surface Plasmon Resonance (SPR) Assays
Key Companies Mentioned: Seegene Inc; DiaSorin SpA; Thermo Fisher Scientific Inc; Bio-Rad Laboratories Inc; Abcam Plc
Countries: Australia; Brazil; China; France; Germany; India; Indonesia; Japan; Russia; South Korea; UK; USA; Canada; Italy; Spain
Regions: Asia-Pacific; Western Europe; Eastern Europe; North America; South America; Middle East; Africa
Time Series: Five years historic and ten years forecast.
Data: Ratios of market size and growth to related markets, GDP proportions, expenditure per capita.
Data Segmentation: Country and regional historic and forecast data, market share of competitors, market segments.
Sourcing and Referencing: Data and analysis throughout the report is sourced using end notes.
Delivery Format: PDF, Word and Excel Data Dashboard.
Companies Mentioned
- Seegene Inc
- DiaSorin SpA
- Thermo Fisher Scientific Inc
- Bio-Rad Laboratories Inc
- Abcam Plc
- PerkinElmer Inc
- Hologic Corporation
- Meso Scale Diagnostics
- Merck KGaA
- Roche Diagnostics India Pvt Ltd
- bioMérieux India
- Becton Dickinson Private Limited
- Danaher Corporation
- Johnson & Johnson
- Simens Healthcare
- Beckman Coulter Inc. India
- Ortho Clinical Diagnostics
- Sysmex Corporation
- Siemens Healthineers Ukraine
- TestLine Clinical Diagnostics s.r.o.
- Cepheid
- F. Hoffmann-La Roche Ltd.
- Hologic
- Bayer AG
- Cantel Medical Corporation
- Meridian Bioscience
- Faizyme Laboratories
- Alere Inc.
- Bangs Laboratories Inc
- The Merck Group
- Takara Bio Inc
- Qiagen
- Spherotech Inc
- Geneaid Biotech Ltd
- Rockland Immunochemicals Inc
- GenScript Biotech Corporation
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 200 |
| Published | March 2025 |
| Forecast Period | 2025 - 2029 |
| Estimated Market Value ( USD | $ 3.92 Billion |
| Forecasted Market Value ( USD | $ 5.42 Billion |
| Compound Annual Growth Rate | 8.4% |
| Regions Covered | Global |
| No. of Companies Mentioned | 36 |