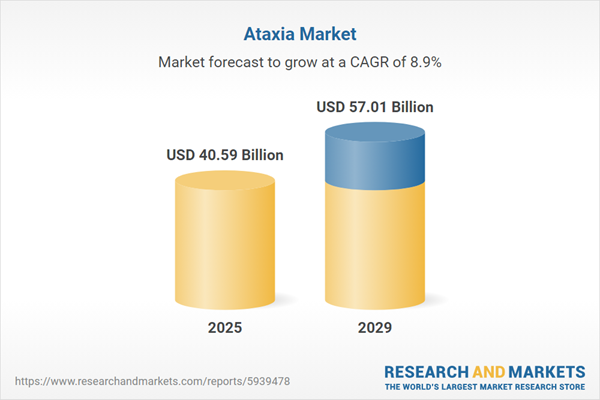
The ataxia market size is expected to see strong growth in the next few years. It will grow to $57.01 billion in 2029 at a compound annual growth rate (CAGR) of 8.9%. The growth in the forecast period can be attributed to accelerated drug development for ataxia, rising investments in rare disease research, adoption of precision medicine approaches, increased focus on patient advocacy, growing understanding of the genetic basis of ataxia. Major trends in the forecast period include integration of digital health solutions in ataxia management, development of gene therapies for ataxia treatment, rise in collaborative research initiatives, emphasis on patient-centric care models, adoption of telemedicine for ataxia consultations.
The increase in alcohol consumption is anticipated to drive the growth of the ataxia market. Alcohol consumption refers to the intake of beverages containing ethanol, typically consumed orally. Cerebellar degeneration, a common form of acquired toxic ataxia, results from chronic alcohol misuse. Patients with alcoholism often experience lower limb postural tremors and gait ataxia. For instance, in August 2024, GOV.UK, a UK government department, reported that the provisional total for Alcohol Duty receipts from wine and other fermented products between May and July 2024 reached £1.15 billion, marking an increase of £32 million (2.9%) compared to the same period the previous year. Therefore, the rise in alcohol consumption is contributing to the growth of the ataxia market.
The upsurge in healthcare expenditure is anticipated to drive the expansion of the ataxia market in the coming years. Elevated healthcare spending encompasses the allocation of resources toward advanced research, development, and accessibility of innovative treatments and therapies targeting ataxia-related conditions. As healthcare expenditures rise, there's a concerted effort to enhance the understanding and management of ataxia. For instance, projections from the 2021-2030 National Health Expenditure (NHE) report, released by the Centers for Medicare & Medicaid Services in March 2022, indicated an average annual increase of 5.1% in national health spending, foreseeing a substantial rise to approximately $6.8 trillion by 2030. Consequently, the upward trajectory in healthcare expenditure acts as a driving force behind the growth observed within the ataxia market.
Innovative product development is a prominent trend gaining momentum within the ataxia market. Leading companies within this domain are committed to introducing novel products or drugs to fortify their market presence. A case in point is the announcement made by Reata Pharmaceuticals in February 2023, unveiling the FDA approval of SKYCLARYS (omavaloxolone). This groundbreaking medication stands as the inaugural drug indicated specifically for individuals with Friedreich's ataxia, encompassing adults and adolescents aged 16 and above. Notably, treatment with SKYCLARYS demonstrated a statistically significant reduction in mFARS (modified Friedreich's Ataxia Rating Scale) scores at Week 48, evidencing diminished impairment compared to a placebo. Friedreich's ataxia, an exceedingly rare inherited neurological disorder, is commonly diagnosed during adolescence.
Major companies in the ataxia market are concentrating on developing innovative solutions, such as drug treatments for ataxia, to improve symptom management and enhance patient outcomes. Ataxia drug treatment consists of medications aimed at managing and alleviating the symptoms of ataxia, a neurological condition marked by impaired coordination, balance, and speech. For instance, in March 2023, Reata Pharmaceuticals, a US-based pharmaceutical company, received FDA approval for SKYCLARYS (omaveloxolone), the first drug specifically indicated for the treatment of Friedreich’s ataxia. This milestone follows promising results from the Phase 2 MOXIe trial, where patients treated with SKYCLARYS demonstrated significantly slower neurological decline compared to those receiving a placebo. The drug activates the Nrf2 protein, crucial for mitochondrial health, addressing a central issue in Friedreich’s ataxia. This approval not only brings hope to patients and their families but also sets a precedent for future treatments of rare diseases.
In December 2022, Solid Biosciences Inc., a US-based biotech company, successfully completed the acquisition of AavantiBio Inc. for an undisclosed amount. This strategic move allows Solid Biosciences Inc. to gain access to AavantiBio's portfolio of neuromuscular and cardiac programs. Notable assets acquired include the differentiated gene transfer candidate SGT-003 for Duchenne, the gene transfer candidate AVB-202 for Friedreich's ataxia, the gene transfer candidate AVB-401 for BAG3-mediated dilated cardiomyopathy, along with additional assets designed for the treatment of unspecified cardiac diseases. AavantiBio Inc. is recognized as a leading gene therapy company in the United States, specializing in the treatment of Friedreich's ataxia and cardiovascular diseases.
Major companies operating in the ataxia market include Pfizer Inc., Johnson & Johnson Services Inc., Eisai Co. Ltd., Genentech Inc., CRISPR Therapeutics AG, Bio-Techne Corporation, Cellectis SA, Eli Lilly & Company, GlaxoSmithKline Plc, Larimar Therapeutics Inc., Capsida Biotherapeutics Inc., Intellia Therapeutics Inc., Bluebird bio Inc., AAVLife SAS, Editas Medicine Inc., Healx, Acorda Therapeutics Inc., H. Lundbeck A/S, Reata Pharmaceuticals Inc., Retrotope Inc., PTC Therapeutics, Zydus Lifesciences Ltd, Ionis Pharmaceuticals Inc, Blade Therapeutics, IntraBio, Biogen Inc., Novartis AG, Pfizer Inc., Sanofi, Roche Holding Ltd., Teva Pharmaceuticals, H. Lundbeck A/S, CRISPR Therapeutics AG, Acorda Therapeutics Inc.
North America was the largest region in the ataxia market in 2024. Asia-Pacific is expected to be the fastest-growing region in the ataxia market report during the forecast period. The regions covered in the ataxia market report are Asia-Pacific, Western Europe, Eastern Europe, North America, South America, Middle East, Africa. The countries covered in the ataxia market report are Australia, Brazil, China, France, Germany, India, Indonesia, Japan, Russia, South Korea, UK, USA, Canada, Italy, Spain.
Ataxia is a neurological disorder characterized by irregular movements and challenges with balance, stemming from a lack of muscular control and coordination. This condition affects various body regions, including limbs, speech, and eye movements.
The primary types of ataxias encompass Friedreich's ataxia, ataxia-telangiectasia, episodic ataxia, and others. Friedreich's ataxia is a rare genetic disorder that disrupts movement and progressively damages the nervous system. Treatment options and diagnostic methods are available for managing this condition. These treatments come in different forms, including solids and liquids, and can be administered through various routes such as oral and parenteral. The end-users for these treatments include hospitals, clinics, home healthcare, and others.
The ataxia market research report is one of a series of new reports that provides ataxia market statistics, including ataxia industry global market size, regional shares, competitors with a ataxia market share, detailed ataxia market segments, market trends and opportunities, and any further data you may need to thrive in the ataxia industry. This ataxia market research report delivers a complete perspective of everything you need, with an in-depth analysis of the current and future scenario of the industry.
The ataxia market consists of revenues earned by entities by providing serotonergic therapy, occupational therapy, vestibular rehabilitation, and hyperbaric oxygen therapy. The market value includes the value of related goods sold by the service provider or included within the service offering. The ataxia market also includes sales of adaptive devices including walkers or canes, acetazolamide, and amantadine. Values in this market are ‘factory gate’ values, that is the value of goods sold by the manufacturers or creators of the goods, whether to other entities (including downstream manufacturers, wholesalers, distributors, and retailers) or directly to end customers. The value of goods in this market includes related services sold by the creators of the goods.
The market value is defined as the revenues that enterprises gain from the sale of goods and/or services within the specified market and geography through sales, grants, or donations in terms of the currency (in USD, unless otherwise specified).
The revenues for a specified geography are consumption values that are revenues generated by organizations in the specified geography within the market, irrespective of where they are produced. It does not include revenues from resales along the supply chain, either further along the supply chain or as part of other products.
This product will be delivered within 3-5 business days.
Table of Contents
Executive Summary
Ataxia Global Market Report 2025 provides strategists, marketers and senior management with the critical information they need to assess the market.This report focuses on ataxia market which is experiencing strong growth. The report gives a guide to the trends which will be shaping the market over the next ten years and beyond.
Reasons to Purchase:
- Gain a truly global perspective with the most comprehensive report available on this market covering 15 geographies.
- Assess the impact of key macro factors such as conflict, pandemic and recovery, inflation and interest rate environment and the 2nd Trump presidency.
- Create regional and country strategies on the basis of local data and analysis.
- Identify growth segments for investment.
- Outperform competitors using forecast data and the drivers and trends shaping the market.
- Understand customers based on the latest market shares.
- Benchmark performance against key competitors.
- Suitable for supporting your internal and external presentations with reliable high quality data and analysis
- Report will be updated with the latest data and delivered to you along with an Excel data sheet for easy data extraction and analysis.
- All data from the report will also be delivered in an excel dashboard format.
Description
Where is the largest and fastest growing market for ataxia? How does the market relate to the overall economy, demography and other similar markets? What forces will shape the market going forward? The ataxia market global report answers all these questions and many more.The report covers market characteristics, size and growth, segmentation, regional and country breakdowns, competitive landscape, market shares, trends and strategies for this market. It traces the market’s historic and forecast market growth by geography.
- The market characteristics section of the report defines and explains the market.
- The market size section gives the market size ($b) covering both the historic growth of the market, and forecasting its development.
- The forecasts are made after considering the major factors currently impacting the market. These include:
- The forecasts are made after considering the major factors currently impacting the market. These include the Russia-Ukraine war, rising inflation, higher interest rates, and the legacy of the COVID-19 pandemic.
- Market segmentations break down the market into sub markets.
- The regional and country breakdowns section gives an analysis of the market in each geography and the size of the market by geography and compares their historic and forecast growth. It covers the growth trajectory of COVID-19 for all regions, key developed countries and major emerging markets.
- The competitive landscape chapter gives a description of the competitive nature of the market, market shares, and a description of the leading companies. Key financial deals which have shaped the market in recent years are identified.
- The trends and strategies section analyses the shape of the market as it emerges from the crisis and suggests how companies can grow as the market recovers.
Scope
Markets Covered:
1) By Type: Friedreich's Ataxia; Ataxia-telangiectasia; Episodic Ataxia; Other Types2) By Product: Treatment; Diagnosis
3) By Dosage Form: Solid; Liquids; Other Dosage Forms
4) By Route of Administration: Oral; Parenteral; Other Routes of Administration
5) By End User: Hospital; Clinics; Home Healthcare; Other End-Users
Subsegments:
1) By Friedreich's Ataxia: Genetic Counseling and Testing; Symptomatic Treatments2) By Ataxia-telangiectasia: Immunotherapy; Supportive Care
3) By Episodic Ataxia: Medications For Episodes; Long-term Management Strategies
4) By Other Types: Spinocerebellar Ataxias (SCAs); Ataxia Due To Vitamin Deficiencies or Toxins
Key Companies Mentioned: Pfizer Inc.; Johnson & Johnson Services Inc.; Eisai Co. Ltd.; Genentech Inc.; CRISPR Therapeutics AG
Countries: Australia; Brazil; China; France; Germany; India; Indonesia; Japan; Russia; South Korea; UK; USA; Canada; Italy; Spain
Regions: Asia-Pacific; Western Europe; Eastern Europe; North America; South America; Middle East; Africa
Time Series: Five years historic and ten years forecast.
Data: Ratios of market size and growth to related markets, GDP proportions, expenditure per capita.
Data Segmentation: Country and regional historic and forecast data, market share of competitors, market segments.
Sourcing and Referencing: Data and analysis throughout the report is sourced using end notes.
Delivery Format: PDF, Word and Excel Data Dashboard.
Companies Mentioned
- Pfizer Inc.
- Johnson & Johnson Services Inc.
- Eisai Co. Ltd.
- Genentech Inc.
- CRISPR Therapeutics AG
- Bio-Techne Corporation
- Cellectis SA
- Eli Lilly & Company
- GlaxoSmithKline Plc
- Larimar Therapeutics Inc.
- Capsida Biotherapeutics Inc.
- Intellia Therapeutics Inc.
- Bluebird bio Inc.
- AAVLife SAS
- Editas Medicine Inc.
- Healx
- Acorda Therapeutics Inc.
- H. Lundbeck A/S
- Reata Pharmaceuticals Inc.
- Retrotope Inc.
- PTC Therapeutics
- Zydus Lifesciences Ltd
- Ionis Pharmaceuticals Inc
- Blade Therapeutics
- IntraBio
- Biogen Inc.
- Novartis AG
- Pfizer Inc.
- Sanofi
- Roche Holding Ltd.
- Teva Pharmaceuticals
- H. Lundbeck A/S
- CRISPR Therapeutics AG
- Acorda Therapeutics Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 200 |
| Published | February 2025 |
| Forecast Period | 2025 - 2029 |
| Estimated Market Value ( USD | $ 40.59 Billion |
| Forecasted Market Value ( USD | $ 57.01 Billion |
| Compound Annual Growth Rate | 8.9% |
| Regions Covered | Global |
| No. of Companies Mentioned | 34 |