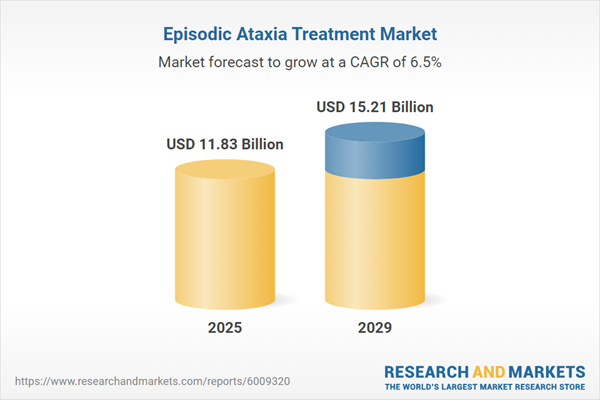
The episodic ataxia treatment market size has grown strongly in recent years. It will grow from $11.07 billion in 2024 to $11.83 billion in 2025 at a compound annual growth rate (CAGR) of 6.9%. The growth in the historic period can be attributed to an increasing understanding of genetic causes, increasing funding for rare disease research, increasing availability of specialized clinics, rising awareness of personalized medicine, and growth in clinical trial participation.
The episodic ataxia treatment market size is expected to see strong growth in the next few years. It will grow to $15.21 billion in 2029 at a compound annual growth rate (CAGR) of 6.5%. The growth in the forecast period can be attributed to increasing genetic testing accessibility, rising demand for genetic counseling services, growing number of clinical trials, increasing number of patient registries, and increasing investment in rare disease biobanks. Major trends in the forecast period include technological advancements, personalized medicine, telemedicine, stem cell therapy, and telerehabilitation services.
The growing prevalence of neurological disorders is anticipated to drive the expansion of the episodic ataxia treatment market. Neurological disorders encompass diseases affecting the central and peripheral nervous systems, including the brain, spinal cord, cranial and peripheral nerves, autonomic nervous system, nerve roots, neuromuscular junction, and muscles. Factors such as an aging population, genetic predispositions, and infectious diseases contribute to the rising incidence of neurological disorders. Episodic ataxia treatment aims to address symptoms and enhance the management of conditions involving intermittent coordination and balance loss. For example, in April 2022, the European Brain Council, a Belgium-based non-profit organization, reported that Dementia (a progressive neurological disorder) impacted 10.5 million people in Europe in 2022, with projections indicating an increase to 18.7 million by 2050. Consequently, the rising prevalence of neurological disorders is fueling the episodic ataxia treatment market.
Leading companies in the episodic ataxia treatment market are prioritizing the development of innovative solutions, such as treatments for Friedreich's ataxia (FA), to improve patient outcomes and address the unmet needs of this rare neurological condition. Episodic ataxia (EA) is characterized by sporadic episodes of ataxia and a lack of voluntary muscle coordination, with episodes varying in duration, frequency, and severity. For instance, in February 2024, Biogen Inc., a US-based biotechnology company, announced the approval of SKYCLARYS (omaveloxolone) for treating Friedreich's ataxia (FA). Omaveloxolone targets the underlying mitochondrial dysfunction associated with FA, potentially altering disease progression rather than merely alleviating symptoms. The therapy functions as a potent transcriptional modulator, aiding in the restoration of mitochondrial function and cellular energy production.
In November 2022, Kriya Therapeutics Inc., a US-based biopharmaceutical company, acquired Redpin Therapeutics Inc. for an undisclosed amount. This acquisition aims to significantly bolster Kriya Therapeutics' neurology pipeline by incorporating Redpin’s innovative treatments for neurological disorders into its existing gene therapy portfolio. Redpin Therapeutics Inc. is a US-based company dedicated to developing treatments for various neurological and neurodegenerative disorders, including episodic ataxia.
Major companies operating in the episodic ataxia treatment market are Pfizer Inc., AbbVie Inc., Sanofi, Bristol-Myers Squibb Company, Novartis AG, Takeda Pharmaceutical Company Limited, Merck KGaA, Banner Health, Astellas Pharma Inc., Daiichi Sankyo Company Limited, UCB S.A., Ipsen, BioMarin Pharmaceutical Inc., Neurocrine Biosciences Inc., PTC Therapeutics Inc., Kissei Pharmaceutical Co. Ltd., Catalyst Pharmaceuticals Inc., Design Therapeutics Inc., Larimar Therapeutics Inc., Voyager Therapeutics Inc., Capsida Biotherapeutics Inc., Adverum Biotechnologies Inc., CRISPR Therapeutics AG.
North America was the largest region in the episodic ataxia treatment market in 2024. Asia-Pacific is expected to be the fastest-growing region in the forecast period. The regions covered in the episodic ataxia treatment market report are Asia-Pacific, Western Europe, Eastern Europe, North America, South America, Middle East, Africa. The countries covered in the episodic ataxia treatment market report are Australia, Brazil, China, France, Germany, India, Indonesia, Japan, Russia, South Korea, UK, USA, Canada, Italy, Spain.
Treatment for episodic ataxia encompasses medical strategies aimed at managing and alleviating the symptoms of this rare neurological disorder, which features intermittent episodes of poor coordination, balance issues, and sometimes vertigo. The approach includes medications, therapies, and lifestyle changes designed to control symptoms and enhance the quality of life for those affected.
The main treatment categories for episodic ataxia include ataxia telangiectasia, episodic ataxia, spinocerebellar ataxia, and Friedreich’s ataxia. Ataxia telangiectasia is a rare genetic disorder impacting both the nervous and immune systems, leading to progressive movement difficulties, an elevated risk of cancer, and other health concerns. Treatments for this condition may involve medications such as levodopa, pramipexole, and venlafaxine, administered through various methods including oral and parenteral routes. Distribution channels for these treatments include direct tenders and retail sales, serving different end users through these channels.
The episodic ataxia treatment market research report is one of a series of new reports that provides episodic ataxia treatment market statistics, including episodic ataxia treatment industry global market size, regional shares, competitors with an episodic ataxia treatment market share, detailed episodic ataxia treatment market segments, market trends and opportunities, and any further data you may need to thrive in the episodic ataxia treatment industry. This episodic ataxia treatment market research report delivers a complete perspective of everything you need, with an in-depth analysis of the current and future scenario of the industry.
The episodic ataxia treatment market consists of revenues earned by entities by providing services such as genetic counseling, physical therapy, and occupational therapy. The market value includes the value of related goods sold by the service provider or included within the service offering. The episodic ataxia treatment market also includes sales of antiepileptic drugs, beta-blockers, genetic testing kits, and wearable health monitors. Values in this market are ‘factory gate’ values, that is, the value of goods sold by the manufacturers or creators of the goods, whether to other entities (including downstream manufacturers, wholesalers, distributors, and retailers) or directly to end customers. The value of goods in this market includes related services sold by the creators of the goods.
The market value is defined as the revenues that enterprises gain from the sale of goods and/or services within the specified market and geography through sales, grants, or donations in terms of the currency (in USD, unless otherwise specified).
The revenues for a specified geography are consumption values that are revenues generated by organizations in the specified geography within the market, irrespective of where they are produced. It does not include revenues from resales along the supply chain, either further along the supply chain or as part of other products.
This product will be delivered within 3-5 business days.
Table of Contents
Executive Summary
Episodic Ataxia Treatment Global Market Report 2025 provides strategists, marketers and senior management with the critical information they need to assess the market.This report focuses on episodic ataxia treatment market which is experiencing strong growth. The report gives a guide to the trends which will be shaping the market over the next ten years and beyond.
Reasons to Purchase:
- Gain a truly global perspective with the most comprehensive report available on this market covering 15 geographies.
- Assess the impact of key macro factors such as conflict, pandemic and recovery, inflation and interest rate environment and the 2nd Trump presidency.
- Create regional and country strategies on the basis of local data and analysis.
- Identify growth segments for investment.
- Outperform competitors using forecast data and the drivers and trends shaping the market.
- Understand customers based on the latest market shares.
- Benchmark performance against key competitors.
- Suitable for supporting your internal and external presentations with reliable high quality data and analysis
- Report will be updated with the latest data and delivered to you along with an Excel data sheet for easy data extraction and analysis.
- All data from the report will also be delivered in an excel dashboard format.
Description
Where is the largest and fastest growing market for episodic ataxia treatment ? How does the market relate to the overall economy, demography and other similar markets? What forces will shape the market going forward? The episodic ataxia treatment market global report answers all these questions and many more.The report covers market characteristics, size and growth, segmentation, regional and country breakdowns, competitive landscape, market shares, trends and strategies for this market. It traces the market’s historic and forecast market growth by geography.
- The market characteristics section of the report defines and explains the market.
- The market size section gives the market size ($b) covering both the historic growth of the market, and forecasting its development.
- The forecasts are made after considering the major factors currently impacting the market. These include: the Russia-Ukraine war, rising inflation, higher interest rates, and the legacy of the COVID-19 pandemic.
- Market segmentations break down the market into sub markets.
- The regional and country breakdowns section gives an analysis of the market in each geography and the size of the market by geography and compares their historic and forecast growth. It covers the growth trajectory of COVID-19 for all regions, key developed countries and major emerging markets.
- The competitive landscape chapter gives a description of the competitive nature of the market, market shares, and a description of the leading companies. Key financial deals which have shaped the market in recent years are identified.
- The trends and strategies section analyses the shape of the market as it emerges from the crisis and suggests how companies can grow as the market recovers.
Scope
Markets Covered:
1) By Type: Ataxia telangiectasia; Episodic ataxia; Spinocerebellar ataxia; Friedreich’s ataxia; Other Types2) By Treatment: Medications; Levodopa; Pramipexole; Venlafaxine; Other Treatments
3) By Route of Administration: Oral; Parenteral; Other Routes of Administration
4) By Distribution Channel: Direct Tender; Retail Sales; Other Distribution Channels
5) By End User: Hospital; Clinics; Home Healthcare; Other End Users
Subsegments
1) By Ataxia Telangiectasia: Neurological Complications; Immunodeficiency; Cancer Susceptibility2) By Episodic Ataxia: Type 1 (EA1); Type 2 (EA2); Other Subtypes
3) By Spinocerebellar Ataxia: Type 1 (SCA1); Type 2 (SCA2); Type 3 (SCA3); Type 6 (SCA6); Type 7 (SCA7); Type 17 (SCA17); Other Types
4) By Friedreich’s Ataxia: Early-onset Friedreich’s Ataxia; Late-onset Friedreich’s Ataxia; Other Types
5) By Other Treatments: Supportive Care (Physical Therapy, Occupational Therapy); Symptomatic Treatment (Muscle Relaxants, Dopaminergic Medications)
Key Companies Mentioned: Pfizer Inc.; AbbVie Inc.; Sanofi; Bristol-Myers Squibb Company; Novartis AG
Countries: Australia; Brazil; China; France; Germany; India; Indonesia; Japan; Russia; South Korea; UK; USA; Canada; Italy; Spain
Regions: Asia-Pacific; Western Europe; Eastern Europe; North America; South America; Middle East; Africa
Time Series: Five years historic and ten years forecast.
Data: Ratios of market size and growth to related markets, GDP proportions, expenditure per capita.
Data Segmentation: Country and regional historic and forecast data, market share of competitors, market segments.
Sourcing and Referencing: Data and analysis throughout the report is sourced using end notes.
Delivery Format: PDF, Word and Excel Data Dashboard.
Companies Mentioned
The major companies featured in this Episodic Ataxia Treatment market report include:- Pfizer Inc.
- AbbVie Inc.
- Sanofi
- Bristol-Myers Squibb Company
- Novartis AG
- Takeda Pharmaceutical Company Limited
- Merck KGaA
- Banner Health
- Astellas Pharma Inc.
- Daiichi Sankyo Company Limited
- UCB S.A.
- Ipsen
- BioMarin Pharmaceutical Inc.
- Neurocrine Biosciences Inc.
- PTC Therapeutics Inc.
- Kissei Pharmaceutical Co. Ltd.
- Catalyst Pharmaceuticals Inc.
- Design Therapeutics Inc.
- Larimar Therapeutics Inc.
- Voyager Therapeutics Inc.
- Capsida Biotherapeutics Inc.
- Adverum Biotechnologies Inc.
- CRISPR Therapeutics AG
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 200 |
| Published | April 2025 |
| Forecast Period | 2025 - 2029 |
| Estimated Market Value ( USD | $ 11.83 Billion |
| Forecasted Market Value ( USD | $ 15.21 Billion |
| Compound Annual Growth Rate | 6.5% |
| Regions Covered | Global |
| No. of Companies Mentioned | 24 |