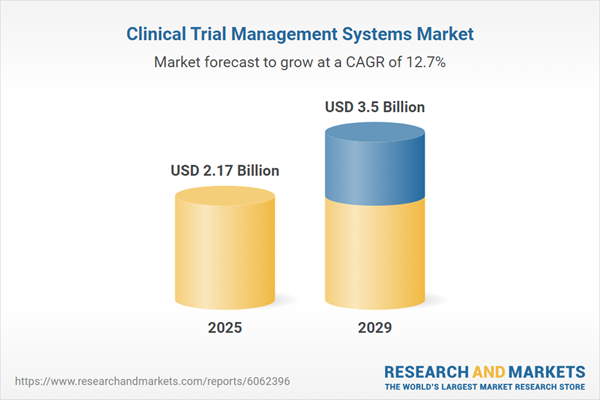
The clinical trial management systems market size has grown rapidly in recent years. It will grow from $1.9 billion in 2024 to $2.17 billion in 2025 at a compound annual growth rate (CAGR) of 14.1%. The growth in the historic period can be attributed to increasing clinical trial complexity, stringent regulatory compliance requirements, focus on data quality and accuracy, rise in outsourcing of clinical trial services.
The clinical trial management systems market size is expected to see rapid growth in the next few years. It will grow to $3.5 billion in 2029 at a compound annual growth rate (CAGR) of 12.7%. The growth in the forecast period can be attributed to integration with electronic health records (EHR), utilization of real-world evidence, adoption of risk-based monitoring, focus on patient-centric approaches, and the emergence of decentralized clinical trials. Major trends in the forecast period include blockchain for data security and integrity, mobile and wearable technology integration, enhanced site management and monitoring capabilities, artificial intelligence (AI) and machine learning (ml) integration, and cloud-based CTMS solutions.
The increasing number of clinical trials is expected to drive the growth of the clinical trial management systems (CTMS) market in the future. Clinical trials are studies that explore new procedures and therapies, assessing their impact on human health. A CTMS is software designed to manage every aspect of clinical trials, including organization, preparation, monitoring, tracking, compliance, and reporting. For example, as of May 2023, a report from Xtalks, a Canada-based magazine, noted that there were 452,604 clinical studies registered on ClinicalTrials.gov, a significant increase from over 365,000 registered trials in early 2021. Therefore, the rising number of clinical trials is propelling the growth of the CTMS market.
The expected growth in the clinical trial management systems market is attributed to the increasing investments in research and development (R&D) within the healthcare sector. R&D investments refer to the financial resources allocated by organizations for discovering, creating, and improving new products, services, processes, or technologies. The expanded investment in R&D activities by pharmaceutical, biotechnology, and medical device companies creates a demand for efficient clinical trial management systems (CTMS) solutions to effectively manage and optimize clinical trials. As an illustration, data from September 2023, provided by the US-based National Center for Science and Engineering Statistics, indicates that in fiscal year 2022, the 42 federally funded research and development centers (FFRDCs) allocated $26.5 billion toward R&D, marking a 6.4% yearly growth in current dollars. Hence, the increasing research and development investments in healthcare are contributing to the growth of the clinical trial management systems market.
Technological advancements are a significant trend gaining traction in the clinical trial management systems market. Major companies in this sector are adopting new technologies to maintain their competitive edge. For example, in June 2024, Medidata, a US-based provider of clinical trial solutions, launched the Medidata Clinical Data Studio. This platform offers a unique, unified system that seamlessly integrates clinical research data from both Medidata and non-Medidata sources, giving stakeholders enhanced control over data quality and facilitating faster, safer trials for patients. By leveraging AI technology, the platform enables study teams to quickly identify potential data issues and safety signals, leading to a more accurate understanding of patients. This integration minimizes the challenges associated with siloed data systems and accelerates data review and reconciliation by up to 80%.
The focus of major companies in the clinical trial management systems market is directed towards the development of innovative solutions, including advanced Clinical Trial Management Systems (CTMS). BGO Software, a Bulgaria-based software company, introduced 'Clinicubes CTMS' in October 2023, representing a sophisticated system designed to streamline and optimize the planning, tracking, management, and reporting of clinical trials. It addresses fundamental study requirements, streamlines critical study processes, provides robust evaluation and standardization features for research specialists, promotes seamless information sharing, ensures prompt delivery of results, guarantees cost-effectiveness, and offers additional benefits.
In August 2024, Valsoft Corporation Inc., a Canada-based company focused on acquiring and developing vertical market software businesses, acquired Anju Software for an undisclosed amount. This acquisition is intended to uphold the strong legacy that Anju has built in the life sciences sector while continuing to develop innovative technology for both existing and new customers. Anju Software is a US-based provider of clinical trial management systems.
Clinical trial management systems (CTMS) are comprehensive cloud-based software platforms designed to oversee clinical studies throughout their entire lifecycle. These systems are commonly utilized for tasks such as clinical trial planning, monitoring, analysis, participant tracking, and financial management.
The primary products in the realm of clinical trial management systems are categorized as enterprise-based and site-based. Enterprise-based systems are substantial software platforms designed to operate within corporate environments, whether in business or government settings. These systems consist of various components, including software and services. Delivery modes for CTMS encompass web-based, on-premise, and cloud-based options, catering to the needs of diverse end-users such as pharmaceutical and biotechnology firms, medical device companies, clinical research organizations (CROs), and other entities.
The clinical trial management systems market research report is one of a series of new reports that provides clinical trial management systems market statistics, including clinical trial management systems industry global market size, regional shares, competitors with a clinical trial management systems market share, detailed clinical trial management systems market segments, market trends and opportunities, and any further data you may need to thrive in the clinical trial management systems industry. This clinical trial management systems market research report delivers a complete perspective of everything you need, with an in-depth analysis of the current and future scenario of the industry.
Major companies operating in the clinical trial management systems market report are International Business Machines Corporation, Thermo Fisher Scientific Inc., Oracle Corporation, Parexel International Corporation, Dassault Systèmes SE, Clario, Veeva Systems Inc., Medpace Holdings Inc., Integron Inc., DSG Inc., ERT Inc., Fortna Inc., DZS Software Solutions Inc., Advarra Inc., Medidata Solutions Inc., ArisGlobal LLC, Anju Software Inc., MasterControl Inc., MedNet Solutions Inc., OmniComm Systems Inc., Bio-Optronics Inc., ClinCapture Inc., Forte Research Systems Inc., DataTRAK International Inc., OpenClinica LLC, GCP-Service International Ltd., SimpleTrials.
North America was the largest region in the clinical trial management systems market in 2024. Asia-Pacific is expected to be the fastest-growing region in the forecast period. The regions covered in the clinical trial management systems market report are Asia-Pacific, Western Europe, Eastern Europe, North America, South America, Middle East, Africa. The countries covered in the clinical trial management systems market report are Australia, Brazil, China, France, Germany, India, Indonesia, Japan, Russia, South Korea, UK, USA, Italy, Spain, Canada.
The clinical trial management systems market includes revenues earned by entities by providing treatment trials, prevention trials, screening trials, supportive and palliative care trials management services. The market value includes the value of related goods sold by the service provider or included within the service offering. Only goods and services traded between entities or sold to end consumers are included.
The market value is defined as the revenues that enterprises gain from the sale of goods and/or services within the specified market and geography through sales, grants, or donations in terms of the currency (in USD, unless otherwise specified).
The revenues for a specified geography are consumption values that are revenues generated by organizations in the specified geography within the market, irrespective of where they are produced. It does not include revenues from resales along the supply chain, either further along the supply chain or as part of other products.
This product will be delivered within 3-5 business days.
Table of Contents
Executive Summary
Clinical Trial Management Systems Global Market Report 2025 provides strategists, marketers and senior management with the critical information they need to assess the market.This report focuses on clinical trial management systems market which is experiencing strong growth. The report gives a guide to the trends which will be shaping the market over the next ten years and beyond.
Reasons to Purchase:
- Gain a truly global perspective with the most comprehensive report available on this market covering 15 geographies.
- Assess the impact of key macro factors such as conflict, pandemic and recovery, inflation and interest rate environment and the 2nd Trump presidency.
- Create regional and country strategies on the basis of local data and analysis.
- Identify growth segments for investment.
- Outperform competitors using forecast data and the drivers and trends shaping the market.
- Understand customers based on the latest market shares.
- Benchmark performance against key competitors.
- Suitable for supporting your internal and external presentations with reliable high quality data and analysis
- Report will be updated with the latest data and delivered to you along with an Excel data sheet for easy data extraction and analysis.
- All data from the report will also be delivered in an excel dashboard format.
Description
Where is the largest and fastest growing market for clinical trial management systems ? How does the market relate to the overall economy, demography and other similar markets? What forces will shape the market going forward? The clinical trial management systems market global report answers all these questions and many more.The report covers market characteristics, size and growth, segmentation, regional and country breakdowns, competitive landscape, market shares, trends and strategies for this market. It traces the market’s historic and forecast market growth by geography.
- The market characteristics section of the report defines and explains the market.
- The market size section gives the market size ($b) covering both the historic growth of the market, and forecasting its development.
- The forecasts are made after considering the major factors currently impacting the market. These include: the Russia-Ukraine war, rising inflation, higher interest rates, and the legacy of the COVID-19 pandemic.
- Market segmentations break down the market into sub markets.
- The regional and country breakdowns section gives an analysis of the market in each geography and the size of the market by geography and compares their historic and forecast growth. It covers the growth trajectory of COVID-19 for all regions, key developed countries and major emerging markets.
- The competitive landscape chapter gives a description of the competitive nature of the market, market shares, and a description of the leading companies. Key financial deals which have shaped the market in recent years are identified.
- The trends and strategies section analyses the shape of the market as it emerges from the crisis and suggests how companies can grow as the market recovers.
Scope
Markets Covered:
1) By Product: Enterprise-Based; Site-Based2) By Components: Software; Services
3) By Delivery Mode: Web-based Clinical Trial Management Systems; on-Premise Clinical Trial Management Systems; Cloud-Based Clinical Trial Management Systems
4) By End-User: Pharmaceutical and Biotechnology Firms; Medical Device Firms; Clinical Research Organization (CROs); Other End-Users
Subsegments:
1) By Enterprise-Based: Cloud-Based; on-Premise2) By Site-Based: Standalone Site; Decentralized Trial Management Systems; Mobile for Site Management
Key Companies Mentioned: International Business Machines Corporation; Thermo Fisher Scientific Inc.; Oracle Corporation; Parexel International Corporation; Dassault Systèmes SE
Countries: Australia; Brazil; China; France; Germany; India; Indonesia; Japan; Russia; South Korea; UK; USA; Canada; Italy; Spain
Regions: Asia-Pacific; Western Europe; Eastern Europe; North America; South America; Middle East; Africa
Time Series: Five years historic and ten years forecast.
Data: Ratios of market size and growth to related markets, GDP proportions, expenditure per capita.
Data Segmentation: Country and regional historic and forecast data, market share of competitors, market segments.
Sourcing and Referencing: Data and analysis throughout the report is sourced using end notes.
Delivery Format: PDF, Word and Excel Data Dashboard.
Companies Mentioned
The major companies featured in this Clinical Trial Management Systems market report include:- International Business Machines Corporation
- Thermo Fisher Scientific Inc.
- Oracle Corporation
- Parexel International Corporation
- Dassault Systèmes SE
- Clario
- Veeva Systems Inc.
- Medpace Holdings Inc.
- Integron Inc.
- DSG Inc.
- ERT Inc.
- Fortna Inc.
- DZS Software Solutions Inc.
- Advarra Inc.
- Medidata Solutions Inc.
- ArisGlobal LLC
- Anju Software Inc.
- MasterControl Inc.
- MedNet Solutions Inc.
- OmniComm Systems Inc.
- Bio-Optronics Inc.
- ClinCapture Inc.
- Forte Research Systems Inc.
- DataTRAK International Inc.
- OpenClinica LLC
- GCP-Service International Ltd.
- SimpleTrials.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 175 |
| Published | April 2025 |
| Forecast Period | 2025 - 2029 |
| Estimated Market Value ( USD | $ 2.17 Billion |
| Forecasted Market Value ( USD | $ 3.5 Billion |
| Compound Annual Growth Rate | 12.7% |
| Regions Covered | Global |
| No. of Companies Mentioned | 28 |