Endoscopes is the fastest growing segment, North America is the largest regional market
Speak directly to the analyst to clarify any post sales queries you may have.
10% Free customizationThis report comes with 10% free customization, enabling you to add data that meets your specific business needs.
These devices include endoscopes, catheters, dialysis machines, lithotripsy systems, and surgical instruments utilized across various urological procedures. The market's expansion is fundamentally driven by the escalating global prevalence of urological disorders, such as benign prostatic hyperplasia, kidney stones, and urinary incontinence, alongside the increasing geriatric population more susceptible to these conditions. The continuous development of minimally invasive surgical techniques also contributes significantly to demand.
Key Market Drivers
The global urology devices market is significantly influenced by the rising prevalence of urological diseases and cancers, which directly translates into a greater need for diagnostic tools, treatment systems, and long-term management solutions. Conditions such as bladder cancer, kidney stones, and benign prostatic hyperplasia necessitate a broad spectrum of interventions, from early detection to complex surgical procedures. For instance, according to the International Agency for Research on Cancer, in August 2023, GLOBOCAN 2022 data estimated 614,298 new bladder cancer cases worldwide in 2022, highlighting the substantial and growing patient population requiring specialized urological care.Key Market Challenges
A critical challenge impeding the growth of the Global Urology Devices Market is the high cost associated with advanced urology devices and procedures. This financial barrier directly hampers market expansion by severely limiting access, particularly in developing regions with constrained healthcare budgets and insufficient reimbursement policies. Elevated costs often compel healthcare systems and individuals to prioritize basic or emergency care, deferring investment in and adoption of newer, more effective diagnostic and therapeutic technologies.Key Market Trends
The expansion of minimally invasive and robotic surgical platforms significantly drives the global urology devices market by offering enhanced precision and improved patient outcomes. These advanced technologies facilitate reduced trauma, shorter hospital stays, and faster recovery. The European Association of Urology's 2024 guidelines noted increasing Minimally Invasive Surgery (MIS) use in paediatric urology, reflecting broader clinical adoption. In March 2024, Intuitive received U. S. Food and Drug Administration clearance for its fifth-generation robotic system, da Vinci 5, initiating a phased launch for enhanced surgical capabilities. This ongoing innovation fuels demand for specialized instruments and integrated robotic systems, profoundly influencing urological surgical practice.Key Market Players Profiled:
- Boston Scientific Corporation
- Fresenius Medical Care AG & Co. KgaA
- Baxter International Inc.
- Becton, Dickinson, and Company
- Olympus Corporation
- B. Braun Melsungen AG
- Medtronic Plc
- Stryker Corporation
- Teleflex Incorporated
- Cook Medical, Inc.
Report Scope:
In this report, the Global Urology Devices Market has been segmented into the following categories:By Type:
- Endoscopes
- Dialysis Devices
- Laser & Lithotripsy Devices
- Endovision & Imaging Devices
- Catheters
- Urodynamic Systems
- Stents
- Biopsy Devices
- Others
By Procedure Type:
- Minimally Invasive Surgery Devices
- Robotic Surgery
By Application:
- Kidney Diseases
- Urological Cancer & BPH
- Pelvic Organ Prolapse
- Others
By End User:
- Hospitals & Clinics
- Ambulatory Care Centers
- Dialysis Centers
- Home Care Settings
By Region:
- North America
- Europe
- Asia-Pacific
- South America
- Middle East & Africa
Competitive Landscape
Company Profiles: Detailed analysis of the major companies present in the Global Urology Devices Market.Available Customizations:
With the given market data, the publisher offers customizations according to a company's specific needs. The following customization options are available for the report.Company Information
- Detailed analysis and profiling of additional market players (up to five).
This product will be delivered within 1-3 business days.
Table of Contents
Companies Mentioned
The companies profiled in this Urology Devices market report include:- Boston Scientific Corporation
- Fresenius Medical Care AG & Co. KgaA
- Baxter International Inc.
- Becton, Dickinson, and Company
- Olympus Corporation
- B. Braun Melsungen AG
- Medtronic Plc
- Stryker Corporation
- Teleflex Incorporated
- Cook Medical, Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 185 |
| Published | November 2025 |
| Forecast Period | 2024 - 2030 |
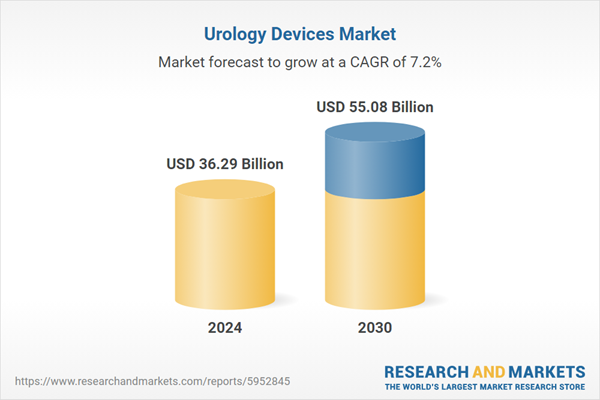
| Estimated Market Value ( USD | $ 36.29 Billion |
| Forecasted Market Value ( USD | $ 55.08 Billion |
| Compound Annual Growth Rate | 7.2% |
| Regions Covered | Global |
| No. of Companies Mentioned | 11 |