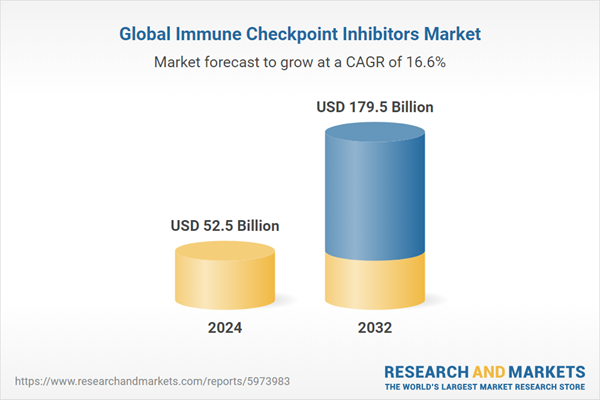
Global Immune Checkpoint Inhibitors Market Analysis
The global immune checkpoint inhibitors market is experiencing rapid growth, fueled by significant advancements in cancer therapy and the increasing prevalence of various cancers globally. Immune checkpoint inhibitors are a class of drugs that block certain proteins made by immune system cells, such as T cells, and some cancer cells. These proteins help keep immune responses in check and can keep T cells from killing cancer cells. When these checkpoints are blocked, T cells can kill cancer cells better.Market Drivers
Several factors are driving the growth of the immune checkpoint inhibitors market. The rising incidence of cancer worldwide necessitates more effective and targeted treatment options, positioning immune checkpoint inhibitors as a critical component in the oncology therapeutics arsenal. Furthermore, the increasing success rates and promising outcomes of immune checkpoint inhibitors in treating a wide range of cancers, such as melanoma, lung cancer, and more recently, cancers of the kidney, bladder, and head and neck, have significantly contributed to their adoption.Advancements in precision medicine and biomarker research have also played a pivotal role. These advancements enable the identification of patients who are most likely to benefit from checkpoint inhibitor therapies, thereby improving treatment efficacy and patient outcomes. Additionally, regulatory support and fast-track approvals for these therapies have accelerated their time-to-market and adoption in clinical settings.
Market Challenges
Despite the market's potential, several challenges need to be addressed. The high cost of immune checkpoint inhibitors poses a significant barrier to access for many patients, particularly in low- and middle-income countries. Moreover, the variability in patient response to these therapies highlights the need for more research into biomarkers and combination therapies to enhance efficacy and predict treatment outcomes more accurately.Another challenge is managing the adverse effects associated with immune checkpoint inhibition, which can range from mild to life-threatening. This necessitates ongoing research and development to understand and mitigate these effects better.
Global Immune Checkpoint Inhibitors Market Trends
The global immune checkpoint inhibitors market is witnessing several transformative trends that are shaping the landscape of cancer therapy. These trends reflect the ongoing evolution in the understanding of cancer immunology and the development of innovative treatments that harness the body's immune system to fight cancer more effectively. Here are the key trends:1. Expansion of Indications
Initially approved for melanoma and non-small cell lung cancer (NSCLC), immune checkpoint inhibitors are now being explored and approved for a broader range of cancer types. This expansion includes indications such as renal cell carcinoma, urothelial carcinoma, head and neck cancers, and hepatocellular carcinoma, among others. The versatility of these drugs across different tumor types underscores their potential in oncology.2. Combination Therapies
There's a growing trend towards combining immune checkpoint inhibitors with other cancer treatments, such as chemotherapy, targeted therapy, and radiation, to enhance treatment efficacy. Clinical trials investigating these combination therapies are showing promising results, indicating that combining modalities can overcome resistance mechanisms and improve patient outcomes.3. Biomarker Development
The development and integration of predictive biomarkers for selecting patients who are most likely to benefit from checkpoint inhibitor therapy are gaining traction. Biomarkers such as PD-L1 expression and tumor mutational burden (TMB) are being used to guide treatment decisions, personalize therapy, and improve clinical outcomes. This trend towards precision medicine is critical for optimizing the use of immune checkpoint inhibitors.4. Regulatory Acceleration and Global Expansion
Regulatory agencies are offering fast-track designations, priority reviews, and accelerated approvals for immune checkpoint inhibitors, particularly for indications with high unmet needs. This regulatory support is speeding the time-to-market for new therapies. Additionally, there's an increased focus on expanding the availability of these treatments in emerging markets, where the burden of cancer is growing.5. Focus on Immune-Related Adverse Events (irAEs)
As the use of immune checkpoint inhibitors becomes more widespread, there's an increasing focus on identifying, managing, and mitigating immune-related adverse events (irAEs) associated with these therapies. Research into understanding the mechanisms behind irAEs and developing strategies to prevent and treat them is crucial for improving patient safety and treatment tolerability.6. Cost and Access Initiatives
Given the high cost of immune checkpoint inhibitors, there are ongoing discussions and initiatives aimed at improving access to these life-saving therapies. This includes pricing negotiations, reimbursement policies, and patient assistance programs, especially in regions where access to advanced cancer treatments is limited.These trends highlight the dynamic nature of the immune checkpoint inhibitors market, driven by scientific advancements, regulatory support, and a deepening understanding of cancer immunotherapy's potential. As research progresses, these trends are expected to continue shaping the development and use of immune checkpoint inhibitors, offering new hope to patients worldwide.
Global Immune Checkpoint Inhibitors Market Segmentation
Market Breakup by Type
- CTLA-4 Inhibitor
- PD-L1 Inhibitor
- PD-1 Inhibitor
- Others
Market Breakup by Application
- Colorectal Cancer
- Hodgkin Lymphoma
- Melanoma
- Bladder Cancer
- Lung Cancer
- Others
Market Breakup by End User
- Hospitals
- Specialty Clinics
- Academic & Research Institutions
- Others
Market Breakup by Region
- North America
- Europe
- Asia Pacific
- Latin America
- Middle East and Africa
Global Immune Checkpoint Inhibitors Market Competitive Landscape
The competitive landscape of the global immune checkpoint inhibitors market is highly dynamic, featuring a mix of established pharmaceutical giants and innovative biotech firms. Companies like Abbvie Inc., BeiGene Ltd, Novartis AG, Incyte Corporation, Regeneron Pharmaceuticals Inc., Pfizer Inc., Merck KGaA, Sanofi, F. Hoffmann-La Roche Ltd., GlaxoSmithKline Plc, Eli Lilly and Company, Bristol-Myers Squibb Company, AstraZeneca PLC and Fortress Biotech Inc. are at the forefront, thanks to their pioneering and market-leading drugs that have transformed cancer treatment paradigms. These companies are contributing to the expanding landscape with their innovative approaches to cancer therapy, showcasing the market's evolution towards more effective and personalized cancer care.Key Questions Answered in This Report
- What is the current and future performance of the global immune checkpoint inhibitors market?
- How are different end-user segments influencing the demand and adoption of immune checkpoint inhibitors in the market?
- How is the application of immune checkpoint inhibitors across various cancer types driving growth and transformation in oncology treatments?
- How are different types of immune checkpoint inhibitors, such as PD-1, PD-L1, and CTLA-4 inhibitors, contributing to the growth and evolution of the oncology market?
- How are established pharmaceutical giants and biotech firms shaping the competitive landscape of the global immune checkpoint inhibitors market?
- What factors are driving the growth of the immune checkpoint inhibitors market across different global regions?
- What are the main players/companies in the market?
Key Benefits for Stakeholders
- The industry report offers a comprehensive quantitative analysis of various market segments, historical and current market trends, market forecasts, and dynamics of the global Immune Checkpoint Inhibitors market from 2017-2032.
- The research report provides the latest information on the market drivers, challenges, and opportunities in the global Immune Checkpoint Inhibitors market.
- The study maps the leading, as well as the fastest-growing, regional markets. It further enables stakeholders to identify the key country-level markets within each region.
- Porter's five forces analysis assists stakeholders in assessing the impact of new entrants, competitive rivalry, supplier power, buyer power, and the threat of substitution. It helps stakeholders to analyze the level of competition within the global Immune Checkpoint Inhibitors industry and its attractiveness.
- The competitive landscape allows stakeholders to understand their competitive environment and provides insight into the current positions of key players in the market.
This product will be delivered within 5-7 business days.
Table of Contents
Companies Mentioned
- Abbvie Inc.
- BeiGene Ltd
- Novartis AG
- Incyte Corporation
- Regeneron Pharmaceuticals Inc.
- Pfizer Inc.
- Merck KGaA
- Sanofi
- F. Hoffmann-La Roche Ltd.
- GlaxoSmithKline Plc
- Eli Lilly
- Bristol-Myers Squibb Company
- AstraZeneca PLC
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 140 |
| Published | May 2024 |
| Forecast Period | 2024 - 2032 |
| Estimated Market Value ( USD | $ 52.5 Billion |
| Forecasted Market Value ( USD | $ 179.5 Billion |
| Compound Annual Growth Rate | 16.6% |
| Regions Covered | Global |
| No. of Companies Mentioned | 13 |