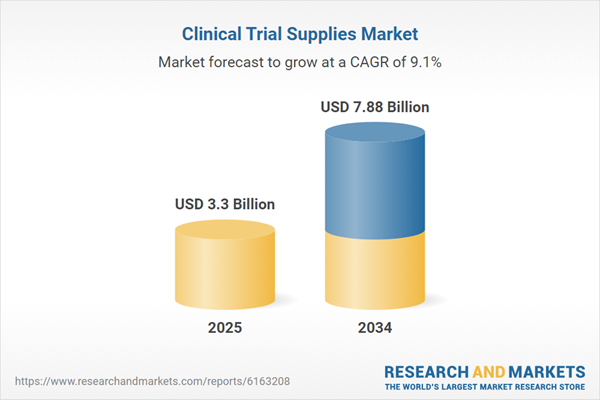
Global Clinical Trial Supplies Market Overview
Clinical trial supplies include tools and equipment used in clinical trials to determine the safety and efficacy of the subject. It can comprise of investigational products, such as drugs, medical devices, or biological substances. To meet quality standards and comply with regulatory guidelines, these supplies need to be managed and maintained appropriately. The increasing incidence of clinical trials to provide desired and effective treatment outcomes to consumers is driving the market growth. Pharmaceutical and biotech companies are increasingly taking part in the drug development process are conducting more clinical trials, propelling market growth.Global Clinical Trial Supplies Market Growth Drivers
Expansion of Decentralised Clinical Trial Solutions for Emerging Biotech Firms
In May 2023, the global biopharmaceutical services provider Allucent partnered with THREAD to launch Allucent Patient Direct Trials, which offers DCT and eCOA technology services. With this strategic partnership, the market witnessed a significant expansion in decentralised clinical trials for companies that are small and emerging. The partnership was intended to bring regulatory expertise technological advancements and development to such companies and facilitate the design and management of customised DCTs. The market is likely to witness substantial companies as Allucent and THREAD assist more small companies to enter the DCT space. The demand for customised digital and logistical support services is expected to escalate as more firms engage in such innovative trial formats. The market is poised to witness an upward trajectory by rising enhancement, speed, and flexibility of clinical research.Standardising Decentralised Clinical Trials to Enhance Efficiency
In June 2023, Medable Inc., a prominent technology provider for patient-centric clinical trials, with the Multi-Regional Clinical Trials Center of Brigham and Women's Hospital and Harvard (MRCT Center), launched an innovative toolkit for Institutional Review Boards (IRBs)/Ethics Committees (ECs) to standardise the ethical review of decentralised clinical trials (DCTs). This innovative toolkit delivered a unified framework, equipped with tools and best practices, aimed at assisting the ethical review and approval procedures, for the ethical conduct of decentralised clinical trials DCTs.As DCTs enable rapid execution of clinical studies, the standardisation facilitated by this toolkit could prevent potential slowdowns due to disjointed and inconsistent ethics reviews, which were identified as critical barriers by the National Institutes of Health and other industry groups. This move is expected to enhance operational efficiency across the global clinical trial supply chain, encouraging wider adoption of DCT methodologies and supporting a shift towards more innovative and responsive clinical research frameworks. Consequently, the clinical trial supplies market is poised to experience significant growth, driven by increased demand for specialised services and supplies aligning with the streamlined DCT models.
Surge in New Launches to Meet Rising Global Clinical Trial Supplies Market Demand
In November 2023, AstraZeneca launched a new venture Evinova intended to provide digital health solutions. AstraZeneca's strategic collaborations with Parexel and Fortrea enabled Evinova to provide scaled digital technology solutions that have been used by AstraZeneca in 40 countries. This new venture aimed at revolutionising the global clinical trials landscape by offering digital health solutions and optimise clinical trial design and delivery. Such advanced technological solutions are likely to escalate market demand for similar solutions, fostering the adoption of home-based trial models. This could result in significant market growth, recording a shift towards more integrated, patient-centric clinical research procedures.Global Clinical Trial Supplies Market Trends
Several trends and developments are being observed in the market to enhance the current situation. Some of the noteworthy trends are as follows:Technological Advancements
The increased adoption of sensors and wearables and the growing influence of patient advocacy groups are contributing directly to the enhanced efficiency of trials.Adoption of Advanced Therapies
The growth of advanced therapy medicinal products (ATMPs), such as gene and cell therapies, presents unique challenges for clinical trial supplies. These therapies often require extremely controlled handling and storage conditions, such as cryogenic temperatures, which necessitate specialised supply solutions.Preference for Personalised Medicine
With increased awareness about medical conditions and potential treatments among healthcare professionals, the market is witnessing a rise in personalised medicine for effective treatments through the genetic profile of individual patients.Increasing Investments
The market is experiencing significant investment activities for the development of software solutions for clinical trials such as decentralised clinical trials. The additional investment in software that supports remote patient monitoring, direct-to-patient logistics, and digital data collection drives market growth.Global Clinical Trial Supplies Market Segmentation
Clinical Trial Supplies Market Report and Forecast 2025-2034 offers a detailed analysis of the market based on the following segments:Market Breakup by Phase
- Phase I
- Phase II
- Phase III
- Others
Market Breakup by Type
- Manufacturing
- Storage and Distribution
- Packaging
- Others
Market Breakup by Therapeutic Use
- Oncology
- CNS
- Cardiovascular
- Infectious Diseases
- Metabolic Disorders
- Others
Market Breakup by End Use
- Pharmaceuticals and Biotechnology Companies
- Contract Research Organisations
- Others
Market Breakup by Region
- North America
- Europe
- Asia Pacific
- Latin America
- Middle East and Africa
Global Clinical Trial Supplies Market Share
Oncology Expected to Dominate the Market Share Based on Therapeutic Use
The therapeutic use segmentation consists of oncology, CNS, cardiovascular, infectious diseases, metabolic disorders, and others. With the increasing prevalence of cancer worldwide, the incidence of oncology clinical trials is on the rise. It is a leading segment as pharmaceutical and biotechnology companies are continuously finding opportunities to discover innovative and effective therapeutics for the treatment of cancer.Pharmaceuticals and Biotechnology Companies, Common End Users of Clinical Trial Supplies
By end users' segmentation, the market is divided into pharmaceutical companies, biotechnology companies, contract research organisations, and others. Out of these, pharmaceutical, and biotechnology companies are the common end users of clinical trial supplies, owing to its crucial role in drug development process. They perform extensive clinical trials to analyse the efficacy and safety of new drugs and therapies. Due to this, a decent supply of materials, including drugs, placebos, and lab equipment is required by these companies contributing to its market growth.Global Clinical Trial Supplies Market Analysis by Region
Based on the regional analysis, the market report covers North America, Europe, Asia Pacific, Latin America, and the Middle East and Africa, with each region contributing to the overall dynamics of the market. North America is leading the regional market with increasing advancements in technologies for making clinical trials more convenient and cost-effective. The presence of a robust healthcare infrastructure and the rising number of clinical trials in the region are propelling the demand for clinical trial supplies in the market. Asia Pacific is following the region and becoming a preferred region for conducting clinical trials by market players. The market growth can be attributed to the presence of a diverse population in the region.Leading Players in the Global Clinical trial Supplies Market
The key features of the market report include funding and investment analysis, and strategic initiatives such as recent partnerships, and collaborations analysis by the leading players. The major companies in the market are as follows:Thermo Fisher Scientific, Inc.
Based out of United States and established in 1956, Thermo Fisher Scientific is a key player of the clinical trials market. It offers a comprehensive range of solutions including packaging, labelling, distribution, and logistics. The platforms offered by the company, further support efficient supply chain of the resources.EUROFINS SCIENTIFIC
Eurofins Scientific is involved in multiple domains and services such as pharmaceutical services, food testing, environmental testing, and clinical diagnostics. It boasts a prominent product portfolio for bioanalytical, microbiology and chemistry testing for drug development and manufacturing, utilising clinical trial supplies effectively.Parexel International (MA) Corporation
Parexel International Corporation was established in 1982 and is headquartered in Massachusetts, USA. The company is one of the leading contract research organisations offering regulatory consulting services to pharmaceutical, medical devices as well as biotechnology market.Other players in the market include Almac Group, Biocair, Catalent, Inc., KLIFO, Movianto, PCI Pharma Services, Sharp Services, LLC, Rubicon Research Pvt. Ltd., Recipharm AB., Piramal Pharma Solutions, NUVISAN GmbH and Myonex
Kindly note that this only represents a partial list of companies, and the complete list has been provided in the report.
Key Queries Solved in the Global Clinical Trial Supplies Market Report
- What was the global clinical trial supplies market value in 2024?
- What is the global clinical trial supplies market forecast outlook for 2025-2034?
- What are the major factors aiding the global market demand?
- What are the major global market trends?
- What is the market segmentation based on the phase?
- What is the market breakup by types?
- How do clinical trials contribute to therapeutic use?
- Who are the end users in the market?
- What are the major markets according to the report?
- Who are the key players in the global clinical trial supplies market?
- How will the market landscape evolve in the upcoming years?
- What are the major drivers, opportunities, and restraints in the market?
- What will be the effect of each driver, challenge, and opportunity on the market?
- Which country is poised to lead the market share in the forecast period?
- Which segment has the highest impact on the market size?
- How are partnerships, collaborations, mergers and acquisitions shaping the market dynamics?
This product will be delivered within 3-5 business days.
Table of Contents
Companies Mentioned
- Almac Group
- Biocair
- Catalent, Inc.
- KLIFO
- Movianto
- PCI Pharma Services
- Sharp Services, LLC
- Parexel International (MA) Corporation
- Myone x
- Rubicon Research Pvt. Ltd.
- Recipharm AB.
- Piramal Pharma Solutions
- NUVISAN GmbH
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 350 |
| Published | July 2025 |
| Forecast Period | 2025 - 2034 |
| Estimated Market Value ( USD | $ 3.3 Billion |
| Forecasted Market Value ( USD | $ 7.88 Billion |
| Compound Annual Growth Rate | 9.1% |
| Regions Covered | Global |
| No. of Companies Mentioned | 13 |