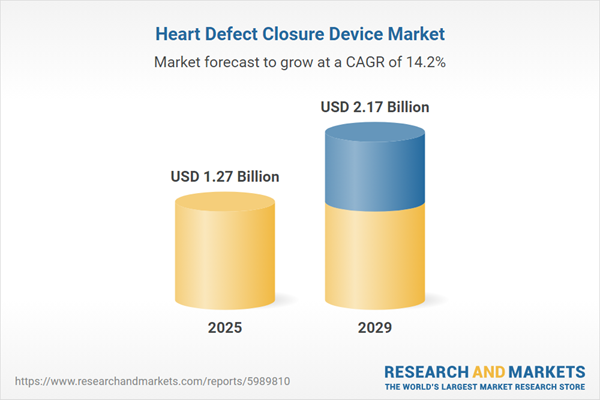
The heart defect closure device market size has grown rapidly in recent years. It will grow from $1.11 billion in 2024 to $1.27 billion in 2025 at a compound annual growth rate (CAGR) of 14.5%. The growth in the historic period can be attributed to growing number of people with congenital heart problems, ageing population, rising health awareness, increasing R&D, and advancements in catheterization techniques.
The heart defect closure device market size is expected to see rapid growth in the next few years. It will grow to $2.17 billion in 2029 at a compound annual growth rate (CAGR) of 14.2%. The growth in the forecast period can be attributed to increasing congenital defects, improved diagnostic techniques, rise in adoption of MRI procedures, rise in use of 3d imaging in heart defect closure procedures, and high occurrence of ischemic stroke. Major trends in the forecast period include technological advancements, development of innovative products, development of next-generation heart closure devices, growing detection of PFO closure devices, and need to assess the benefits of PFO closure for the aged population.
The increasing prevalence of congenital heart problems is expected to drive the growth of the heart defect closure device market in the coming years. Congenital heart problems are structural defects in the heart present from birth. The rise in congenital heart issues can be attributed to factors such as genetic predispositions, environmental influences, and maternal health conditions like diabetes, obesity, and exposure to certain medications or infections during pregnancy. Heart defect closure devices provide minimally invasive solutions to treat these conditions by sealing abnormal heart openings, restoring normal blood flow, reducing complications, and enhancing overall cardiac function. For example, in October 2024, an article published in the National Library of Medicine (NLM), a US-based biomedical research organization, reported a sharp increase in the incidence rate of congenital heart disease (CHD) to 5.46% in 2023, up from 1.12% in 2020, 2.36% in 2021, and 3.87% in 2022. As a result, the growing number of individuals with congenital heart problems is driving the expansion of the heart defect closure device market.
Major companies in the heart defect closure device market are developing self-expanding, double-disc occluders to provide minimally invasive solutions for sealing heart defects. These occluders enhance procedural safety, improve patient outcomes by reducing recovery times, and minimize the risk of complications associated with traditional open-heart surgeries. For example, in September 2022, Abbott Laboratories, a US-based medical devices and healthcare company, launched the Amplatzer Talisman PFO Occlusion System in Europe. This device is designed to close a patent foramen ovale (PFO), an abnormal opening between the left and right atria of the heart that can lead to strokes and other complications. The system uses a minimally invasive procedure to deploy a self-expanding, double-disc occluder made of nitinol wire mesh, effectively sealing the PFO and preventing the passage of clots between the atria.
In February 2023, Boston Scientific Corporation, a US-based medical device manufacturer, acquired a 65% stake in Acotec Scientific Holdings Limited for $0.523 billion. This acquisition enhances Boston Scientific Corporation’s position in China and enables the global commercialization of Acotec Scientific Holdings Limited’s products. Acotec Scientific Holdings Limited is a China-based medical device company specializing in the interventional treatment of vascular diseases.
Major players in the heart defect closure device market are Abbott Laboratories, Medtronic, Koninklijke Philips N.V., Boston Scientific Corporation, Terumo Corporation, Edwards Lifesciences, W L Gore and Associates, B. Braun, BIOTRONIK SE & Co. KG, Lepu Medical Technology, Meril Life Sciences Pvt. Ltd., MicroPort Scientific Corporation, AtriCure Inc., LifeTech Scientific Corporation, Baylis Medical Company Inc., Biosense Webster Inc., Sahajanand Medical Technologies Limited, Occlutech, Coherex Medical Inc., Cardia Inc., Acrostak Corporation, Vivasure Medical, Transmural Systems Inc., Cormatrix Cardiovascular Inc., and St. Jude Medical LLC.
North America was the largest region in the heart defect closure device market in 2024. Asia-Pacific is expected to be the fastest-growing region in the forecast period. The regions covered in heart defect closure device report are Asia-Pacific, Western Europe, Eastern Europe, North America, South America, Middle East and Africa. The countries covered in the heart defect closure device market report are Australia, Brazil, China, France, Germany, India, Indonesia, Japan, Russia, South Korea, UK, USA, Canada, Italy, Spain.
A heart defect closure device is a medical instrument designed to close abnormal openings or defects in the heart's structure, particularly in the septum, the wall that separates the heart's chambers. These devices are used to treat specific heart conditions, such as atrial septal defects or patent foramen ovale, by sealing the openings in the heart's wall. They are implanted through minimally invasive procedures, offering an effective and less invasive alternative to open-heart surgery for patients with these defects.
The primary types of heart defect closure devices include atrial septal defect (ASD) closure devices, left atrial appendage (LAA) closure devices, patent foramen ovale (PFO) closure devices, patent ductus arteriosus (PDA) closure devices, and ventricular septal defect (VSD) closure devices. An ASD closure device is specifically designed to seal a hole in the wall between the heart's two upper chambers. These devices are constructed from various materials such as nitinol and stainless steel and can be delivered through transcatheter, surgical, and other delivery methods by hospitals, clinics, ambulatory surgical centers, and other healthcare facilities.
The heart defect closure device market research report is one of a series of new reports that provides heart defect closure device market statistics, including the heart defect closure device industry global market size, regional shares, competitors with heart defect closure device market share, detailed heart defect closure device market segments, market trends, and opportunities, and any further data you may need to thrive in the heart defect closure device industry. These heart defect closure device market research reports deliver a complete perspective of everything you need, with an in-depth analysis of the current and future scenarios of the industry.
The heart defect closure device market consists of sales of amplatzer septal occluder, gore cardioform septal occluder, and HELEX septal occluder. Values in this market are ‘factory gate’ values, that is, the value of goods sold by the manufacturers or creators of the goods, whether to other entities (including downstream manufacturers, wholesalers, distributors, and retailers) or directly to end customers. The value of goods in this market includes related services sold by the creators of the goods.
The market value is defined as the revenues that enterprises gain from the sale of goods and/or services within the specified market and geography through sales, grants, or donations in terms of the currency (in USD, unless otherwise specified).
The revenues for a specified geography are consumption values that are revenues generated by organizations in the specified geography within the market, irrespective of where they are produced. It does not include revenues from resales along the supply chain, either further along the supply chain or as part of other products.
This product will be delivered within 3-5 business days.
Table of Contents
Executive Summary
Heart Defect Closure Device Global Market Report 2025 provides strategists, marketers and senior management with the critical information they need to assess the market.This report focuses on heart defect closure device market which is experiencing strong growth. The report gives a guide to the trends which will be shaping the market over the next ten years and beyond.
Reasons to Purchase:
- Gain a truly global perspective with the most comprehensive report available on this market covering 15 geographies.
- Assess the impact of key macro factors such as conflict, pandemic and recovery, inflation and interest rate environment and the 2nd Trump presidency.
- Create regional and country strategies on the basis of local data and analysis.
- Identify growth segments for investment.
- Outperform competitors using forecast data and the drivers and trends shaping the market.
- Understand customers based on the latest market shares.
- Benchmark performance against key competitors.
- Suitable for supporting your internal and external presentations with reliable high quality data and analysis
- Report will be updated with the latest data and delivered to you along with an Excel data sheet for easy data extraction and analysis.
- All data from the report will also be delivered in an excel dashboard format.
Description
Where is the largest and fastest growing market for heart defect closure device ? How does the market relate to the overall economy, demography and other similar markets? What forces will shape the market going forward? The heart defect closure device market global report answers all these questions and many more.The report covers market characteristics, size and growth, segmentation, regional and country breakdowns, competitive landscape, market shares, trends and strategies for this market. It traces the market’s historic and forecast market growth by geography.
- The market characteristics section of the report defines and explains the market.
- The market size section gives the market size ($b) covering both the historic growth of the market, and forecasting its development.
- The forecasts are made after considering the major factors currently impacting the market. These include: the Russia-Ukraine war, rising inflation, higher interest rates, and the legacy of the COVID-19 pandemic.
- Market segmentations break down the market into sub markets.
- The regional and country breakdowns section gives an analysis of the market in each geography and the size of the market by geography and compares their historic and forecast growth. It covers the growth trajectory of COVID-19 for all regions, key developed countries and major emerging markets.
- The competitive landscape chapter gives a description of the competitive nature of the market, market shares, and a description of the leading companies. Key financial deals which have shaped the market in recent years are identified.
- The trends and strategies section analyses the shape of the market as it emerges from the crisis and suggests how companies can grow as the market recovers.
Scope
Markets Covered:
1) By Type: Atrial Septal Defect (ASD) Closure Device; Left Atrial Appendage (LAA) Closure Devices; Patent Foramen Ovale (PFO) Closure Devices; Patent Ductus Arteriosus (PDA) Closure Devices; Ventricular Septal Defect (VSD) Closure Devices2) By Material: Nitinol-Based Devices; Stainless Steel Devices; Other Materials
3) By Mode of Delivery: Transcatheter Delivery; Surgical Delivery; Other Modes
4) By End-User: Hospitals; Clinics; Ambulatory Surgical Centers; Other End-Users
Subsegments:
1) By Atrial Septal Defect (ASD) Closure Device: Occluder Devices (Septal Occluders); Implantable Patch Devices; Self-Expanding Devices; Balloon-Expandable Devices2) By Left Atrial Appendage (LAA) Closure Devices: Occluder Devices (WATCHMAN Device); Clip-Based Devices; Self-Expanding LAA Closure Devices
3) By Patent Foramen Ovale (PFO) Closure Devices: Septal Occluders; Self-Expanding Occluders; Balloon-Expandable Occluders
4) By Patent Ductus Arteriosus (PDA) Closure Devices: Coil-Based Closure Devices; Stent-Based Closure Devices; Amplatzer Occluders
5) By Ventricular Septal Defect (VSD) Closure Devices: Septal Occluder Devices; Balloon-Expandable Occluders; Self-Expanding Occluders
Key Companies Mentioned: Abbott Laboratories; Medtronic; Koninklijke Philips N.V.; Boston Scientific Corporation; Terumo Corporation
Countries: Australia; Brazil; China; France; Germany; India; Indonesia; Japan; Russia; South Korea; UK; USA; Canada; Italy; Spain
Regions: Asia-Pacific; Western Europe; Eastern Europe; North America; South America; Middle East; Africa
Time Series: Five years historic and ten years forecast.
Data: Ratios of market size and growth to related markets, GDP proportions, expenditure per capita.
Data Segmentation: Country and regional historic and forecast data, market share of competitors, market segments.
Sourcing and Referencing: Data and analysis throughout the report is sourced using end notes.
Delivery Format: PDF, Word and Excel Data Dashboard.
Companies Mentioned
The major companies featured in this Heart Defect Closure Device market report include:- Abbott Laboratories
- Medtronic
- Koninklijke Philips N.V.
- Boston Scientific Corporation
- Terumo Corporation
- Edwards Lifesciences
- W L Gore and Associates
- B. Braun
- BIOTRONIK SE & Co. KG
- Lepu Medical Technology
- Meril Life Sciences Pvt. Ltd.
- MicroPort Scientific Corporation
- AtriCure Inc.
- LifeTech Scientific Corporation
- Baylis Medical Company Inc.
- Biosense Webster Inc.
- Sahajanand Medical Technologies Limited
- Occlutech
- Coherex Medical Inc.
- Cardia Inc.
- Acrostak Corporation
- Vivasure Medical
- Transmural Systems Inc.
- Cormatrix Cardiovascular Inc.
- St. Jude Medical LLC
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 200 |
| Published | April 2025 |
| Forecast Period | 2025 - 2029 |
| Estimated Market Value ( USD | $ 1.27 Billion |
| Forecasted Market Value ( USD | $ 2.17 Billion |
| Compound Annual Growth Rate | 14.2% |
| Regions Covered | Global |
| No. of Companies Mentioned | 26 |