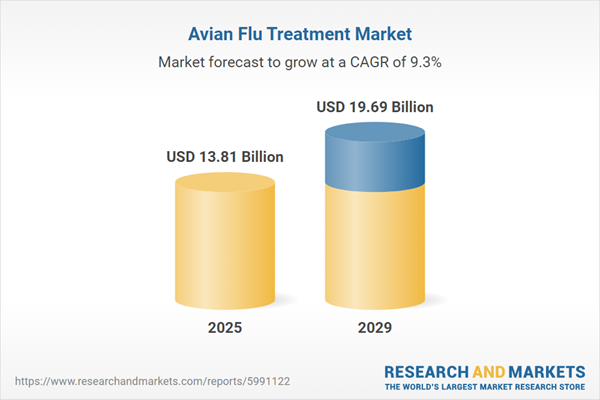
The avian flu treatment market size has grown strongly in recent years. It will grow from $12.59 billion in 2024 to $13.81 billion in 2025 at a compound annual growth rate (CAGR) of 9.7%. The growth in the historic period can be attributed to increased demand for avian flu treatment, rise in measures to prevent the spread of bird flu, increased availability of treatment options, increased investment for healthcare infrastructure, and increased demand for poultry for consumption.
The avian flu treatment market size is expected to see strong growth in the next few years. It will grow to $19.69 billion in 2029 at a compound annual growth rate (CAGR) of 9.3%. The growth in the forecast period can be attributed to growing awareness and diagnosis, government initiatives for pandemic preparedness, rising research and development investments, the need for effective therapeutics against avian influenza, and extensive research and development efforts. Major trends in the forecast period include the development of novel therapeutic approaches, future innovation and technologies, collaborative research efforts, new business development, and investment opportunities, and telemedicine and remote consultation implementation.
Growing awareness and improved diagnosis are anticipated to drive the expansion of the avian flu treatment market in the future. Awareness involves recognizing a condition, while diagnosis identifies a specific issue based on that awareness. The demand for avian flu treatment stems from the urgent need to protect public health, prevent potential pandemics, and shield poultry populations from substantial economic losses. Awareness and early diagnosis are vital for effective avian flu treatment, enabling prompt intervention and containment to prevent the disease from spreading. For instance, the World Health Organization (WHO), a Switzerland-based intergovernmental organization, reported in April 2024 that over 8,000 people in the US were under active surveillance from February 9, 2022, to March 29, 2024, after exposure to animals believed to be infected with the HPAI A (H5N1) virus. Thus, increasing awareness and diagnosis are driving the growth of the avian flu treatment market.
Leading companies in the avian flu treatment market are emphasizing partnerships to improve efficacy and accelerate the development of new treatments. These partnerships involve collaborations between pharmaceutical companies, research institutions, and government agencies to pool resources, share expertise, and conduct innovative clinical trials. For example, in April 2024, CureVac N.V., a biotechnology company based in Germany, partnered with GSK plc, a UK-based pharmaceutical company, to develop an investigational influenza A (H5N1) pre-pandemic vaccine candidate. This collaboration aims to address the potential future pandemic threat posed by the H5N1 avian influenza virus by evaluating the safety, reactogenicity, and immunogenicity of the monovalent vaccine candidate, which uses CureVac's proprietary second-generation mRNA technology. The combined Phase 1/2 study will assess the vaccine candidate's safety and effectiveness in healthy adults aged 18 to 64 and older adults aged 65 to 85.
In December 2022, Hester Biosciences Ltd., an Indian animal and poultry vaccine manufacturing company, acquired technology from the Indian Council of Agricultural Research and the National Institute of High-Security Animal Diseases (ICAR-NIHSAD) for an undisclosed amount. This acquisition aims to develop an inactivated H9N2 avian influenza vaccine for poultry, providing a solution to the significant economic losses experienced by Indian poultry farmers due to periodic outbreaks of the disease. The Indian Council of Agricultural Research and the National Institute of High-Security Animal Diseases (ICAR-NIHSAD) is an India-based research institute that offers technology for avian flu vaccines and diagnostic tests for avian influenza.
Major companies operating in the avian flu treatment market are Pfizer Inc., F. Hoffmann-La Roche Ltd., Merck & Co. Inc., Sanofi S.A., AstraZeneca Plc, Novartis AG, GlaxoSmithKline Plc, Gilead Sciences Inc., Baxter International Inc., CSL Limited, Moderna Inc., Cipla Ltd., Shionogi & Co. Ltd., Novavax Inc., Seqirus Pty Ltd., Sinovac Biotech Ltd., Emergent BioSolutions Inc., Dynavax Technologies Corporation, CureVac AG, Valneva SE, BioCryst Pharmaceuticals Inc., Siga Technologies Inc., Aridis Pharmaceuticals Inc., Inovio Pharmaceuticals Inc., Biolyse Pharma.
North America was the largest region in the avian flu treatment market in 2024. Asia-Pacific is expected to be the fastest growing region in the market. The regions covered in the avian flu treatment market report are Asia-Pacific, Western Europe, Eastern Europe, North America, South America, Middle East, Africa. The countries covered in the avian flu treatment market report are Australia, Brazil, China, France, Germany, India, Indonesia, Japan, Russia, South Korea, UK, USA, Canada, Italy, Spain.
Avian flu treatment involves medical approaches and strategies aimed at managing and easing symptoms of avian influenza, commonly known as bird flu, in infected individuals. Avian influenza is caused by viruses that primarily affect birds, though certain strains such as H5N1 and H7N9 can also infect humans, leading to severe illness or even death in some cases.
The primary treatments for avian flu include antibiotics, vaccines, antiviral agents, and immunoglobulins. Antibiotics are medications designed to combat bacterial infections. These treatments are accessible through both online and offline pharmacies and are utilized by various end-users such as hospitals, institutional health centers, and clinics.
The avian flu treatment market research report is one of a series of new reports that provides avian flu treatment market statistics, including avian flu treatment industry global market size, regional shares, competitors with a avian flu treatment market share, detailed avian flu treatment market segments, market trends and opportunities, and any further data you may need to thrive in the avian flu treatment industry. This avian flu treatment market research report delivers a complete perspective of everything you need, with an in-depth analysis of the current and future scenario of the industry.
The avian flu treatment market consists of sales of antiviral medications, respiratory support equipment, and intravenous fluids. Values in this market are ‘factory gate’ values, that is the value of goods sold by the manufacturers or creators of the goods, whether to other entities (including downstream manufacturers, wholesalers, distributors and retailers) or directly to end customers. The value of goods in this market includes related services sold by the creators of the goods.
The market value is defined as the revenues that enterprises gain from the sale of goods and/or services within the specified market and geography through sales, grants, or donations in terms of the currency (in USD, unless otherwise specified).
The revenues for a specified geography are consumption values that are revenues generated by organizations in the specified geography within the market, irrespective of where they are produced. It does not include revenues from resales along the supply chain, either further along the supply chain or as part of other products.
This product will be delivered within 3-5 business days.
Table of Contents
Executive Summary
Avian Flu Treatment Global Market Report 2025 provides strategists, marketers and senior management with the critical information they need to assess the market.This report focuses on avian flu treatment market which is experiencing strong growth. The report gives a guide to the trends which will be shaping the market over the next ten years and beyond.
Reasons to Purchase:
- Gain a truly global perspective with the most comprehensive report available on this market covering 15 geographies.
- Assess the impact of key macro factors such as conflict, pandemic and recovery, inflation and interest rate environment and the 2nd Trump presidency.
- Create regional and country strategies on the basis of local data and analysis.
- Identify growth segments for investment.
- Outperform competitors using forecast data and the drivers and trends shaping the market.
- Understand customers based on the latest market shares.
- Benchmark performance against key competitors.
- Suitable for supporting your internal and external presentations with reliable high quality data and analysis
- Report will be updated with the latest data and delivered to you along with an Excel data sheet for easy data extraction and analysis.
- All data from the report will also be delivered in an excel dashboard format.
Description
Where is the largest and fastest growing market for avian flu treatment ? How does the market relate to the overall economy, demography and other similar markets? What forces will shape the market going forward? The avian flu treatment market global report answers all these questions and many more.The report covers market characteristics, size and growth, segmentation, regional and country breakdowns, competitive landscape, market shares, trends and strategies for this market. It traces the market’s historic and forecast market growth by geography.
- The market characteristics section of the report defines and explains the market.
- The market size section gives the market size ($b) covering both the historic growth of the market, and forecasting its development.
- The forecasts are made after considering the major factors currently impacting the market. These include: the Russia-Ukraine war, rising inflation, higher interest rates, and the legacy of the COVID-19 pandemic.
- Market segmentations break down the market into sub markets.
- The regional and country breakdowns section gives an analysis of the market in each geography and the size of the market by geography and compares their historic and forecast growth. It covers the growth trajectory of COVID-19 for all regions, key developed countries and major emerging markets.
- The competitive landscape chapter gives a description of the competitive nature of the market, market shares, and a description of the leading companies. Key financial deals which have shaped the market in recent years are identified.
- The trends and strategies section analyses the shape of the market as it emerges from the crisis and suggests how companies can grow as the market recovers.
Scope
Markets Covered:
1) By Type: Antibiotics; Vaccines; Antiviral Agents; Immunoglobulins2) By Distribution Channel: Online Pharmacies; Offline Pharmacies
3) By End User: Hospital; Institutional Health Centers; Clinics
Subsegments:
1) By Antibiotics: Broad-Spectrum Antibiotics; Tetracycline Antibiotics; Penicillin-Based Antibiotics; Macrolide Antibiotics; Cephalosporins; Antimicrobial Peptides2) By Vaccines: Inactivated Vaccines; Live Attenuated Vaccines; Recombinant Vaccines; Subunit Vaccines; Dna Vaccines; Bivalent and Multivalent Vaccines
3) By Antiviral Agents: Neuraminidase Inhibitors (Oseltamivir, Zanamivir); M2 Ion Channel Inhibitors (Amantadine); Polymerase Inhibitors; Protease Inhibitors; Antiviral Combination Therapies
4) By Immunoglobulins: Human Immunoglobulin (Ivig); Monoclonal Antibodies; Hyperimmune Serum; Polyvalent Immunoglobulins; Avian Flu-Specific Immunoglobulins
Key Companies Mentioned: Pfizer Inc.; F. Hoffmann-La Roche Ltd.; Merck & Co. Inc.; Sanofi S.a.; AstraZeneca Plc
Countries: Australia; Brazil; China; France; Germany; India; Indonesia; Japan; Russia; South Korea; UK; USA; Canada; Italy; Spain
Regions: Asia-Pacific; Western Europe; Eastern Europe; North America; South America; Middle East; Africa
Time Series: Five years historic and ten years forecast.
Data: Ratios of market size and growth to related markets, GDP proportions, expenditure per capita.
Data Segmentation: Country and regional historic and forecast data, market share of competitors, market segments.
Sourcing and Referencing: Data and analysis throughout the report is sourced using end notes.
Delivery Format: PDF, Word and Excel Data Dashboard.
Companies Mentioned
The major companies featured in this Avian Flu Treatment market report include:- Pfizer Inc.
- F. Hoffmann-La Roche Ltd.
- Merck & Co. Inc.
- Sanofi S.A.
- AstraZeneca Plc
- Novartis AG
- GlaxoSmithKline Plc
- Gilead Sciences Inc.
- Baxter International Inc.
- CSL Limited
- Moderna Inc.
- Cipla Ltd.
- Shionogi & Co. Ltd.
- Novavax Inc.
- Seqirus Pty Ltd.
- Sinovac Biotech Ltd.
- Emergent BioSolutions Inc.
- Dynavax Technologies Corporation
- CureVac AG
- Valneva SE
- BioCryst Pharmaceuticals Inc.
- Siga Technologies Inc.
- Aridis Pharmaceuticals Inc.
- Inovio Pharmaceuticals Inc.
- Biolyse Pharma
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 200 |
| Published | April 2025 |
| Forecast Period | 2025 - 2029 |
| Estimated Market Value ( USD | $ 13.81 Billion |
| Forecasted Market Value ( USD | $ 19.69 Billion |
| Compound Annual Growth Rate | 9.3% |
| Regions Covered | Global |
| No. of Companies Mentioned | 26 |