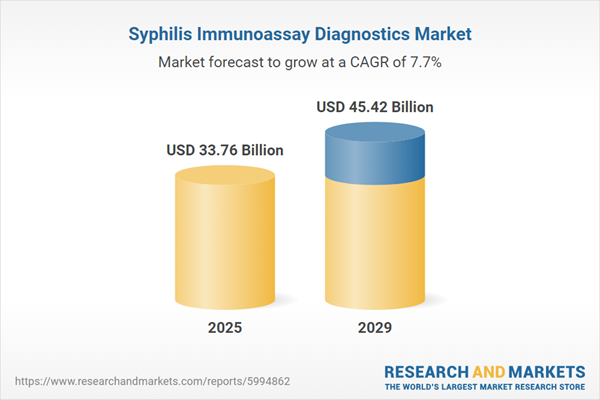
The syphilis immunoassay diagnostics market size has grown strongly in recent years. It will grow from $31.25 billion in 2024 to $33.76 billion in 2025 at a compound annual growth rate (CAGR) of 8%. The growth in the historic period can be attributed to rise in socioeconomic factors such as STDs, increasing percentage of unidentified sex, growing incidences of fatal syphilis diseases, growing initiatives by the government, and rising emphasis on early detection of the diseases.
The syphilis immunoassay diagnostics market size is expected to see strong growth in the next few years. It will grow to $45.42 billion in 2029 at a compound annual growth rate (CAGR) of 7.7%. The growth in the forecast period can be attributed to rapid growth of high-risk population, increasing awareness about partner notification programs, rise in the prevalence, increase awareness of syphilis immunoassay diagnostics, and surge in government expenditure on healthcare. Major trends in the forecast period include adoption of innovative technologies, development of rapid point-of-care tests, growing demand for home test kits, advancements in laboratory testing methods, and expansion of the diagnostic laboratory techniques.
The increasing prevalence of sexually transmitted diseases (STDs) is expected to drive the growth of the syphilis immunoassay diagnostics market in the coming years. STDs, or sexually transmitted infections (STIs), are primarily spread through sexual contact, including vaginal, anal, and oral sex. The rise in STDs can be attributed to factors such as reduced condom use, antibiotic resistance, lack of sexual education, changes in social and behavioral patterns, stigma, reduced funding for STD prevention programs, and greater travel. Syphilis immunoassay diagnostics play a critical role in the detection and management of STDs by providing accurate and timely identification of syphilis infections, which enables appropriate treatment and preventive measures. For example, in June 2024, the UK-based Terrence Higgins Trust reported that data from the UK Health Security Agency (UKHSA) indicated 401,800 STIs were reported in England in 2023, a 5% increase from the previous year. As a result, the growing incidence of STDs is fueling the demand for syphilis immunoassay diagnostics.
Major companies in the syphilis immunoassay diagnostics market are prioritizing the development of innovative solutions, including rapid syphilis tests, to enhance the efficiency and accuracy of syphilis detection. Rapid syphilis tests are user-friendly, point-of-care tools that detect syphilis antibodies in blood or bodily fluids within minutes, facilitating prompt diagnosis and early treatment. For example, in February 2023, Chembio Diagnostics Inc., a US-based company specializing in point-of-care diagnostics for infectious diseases, received a CLIA waiver from the FDA for its DPP HIV-Syphilis System. This dual rapid test is designed to simultaneously detect antibodies to HIV 1/2 and Treponema pallidum, the bacterium that causes syphilis. Conducted in just 15 minutes, this test aims to enhance diagnostic accuracy and treatment efficacy, particularly in preventing mother-to-child transmission of syphilis and reducing the risk of HIV transmission among individuals with syphilis.
In April 2023, Biosynex SA, a France-based company specializing in syphilis immunoassay diagnostics, acquired Chembio Diagnostics, Inc. for $17.2 million. This strategic acquisition aims to bolster Biosynex's market footprint in North America by leveraging Chembio's established portfolio of FDA-approved products and expertise in rapid diagnostic tests. Chembio Diagnostics Inc., based in the United States, is renowned for its focus on point-of-care diagnostics for infectious diseases, including syphilis.
Major companies operating in the syphilis immunoassay diagnostics market are F. Hoffmann-La Roche Ltd., Thermo Fisher Scientific Inc., Abbott Laboratories, Siemens Healthineers, Becton Dickinson and Company, bioMérieux SA, Bio-Rad Laboratories Inc., Meril Life Sciences Pvt. Ltd., Randox Laboratories Ltd., SEKISUI Diagnostics, Bloodworks Northwest, LetsGetChecked, Everlywell Inc., ACON Laboratories Inc., Trinity Biotech, Fujirebio, CTK Biotech Inc., bioLytical Laboratories Inc., AdvaCare Pharma, MyBioSource.com, Gold Standard Diagnostics Frankfurt GmbH, Newmarket Biomedical Ltd, InTec PRODUCTS INC., NuGenerex Diagnostics LLC.
North America was the largest region in the syphilis immunoassay diagnostics market in 2024. Asia-Pacific is expected to be the fastest-growing region in the forecast period. The regions covered in the syphilis immunoassay diagnostics market report are Asia-Pacific, Western Europe, Eastern Europe, North America, South America, Middle East, Africa. The countries covered in the syphilis immunoassay diagnostics market report are Australia, Brazil, China, France, Germany, India, Indonesia, Japan, Russia, South Korea, UK, USA, Canada, Italy, Spain.
Syphilis immunoassay diagnostics refer to laboratory tests designed to detect antibodies or antigens associated with the bacterium treponema pallidum, the causative agent of syphilis. These diagnostics utilize immunoassay techniques to detect specific immune responses present in the blood. The primary objective of syphilis immunoassay diagnostics is to accurately diagnose syphilis infections, facilitating prompt and effective treatment.
Key products in syphilis immunoassay diagnostics include analyzers, reagents, kits, and related items. Analyzers are devices employed in laboratories to analyze samples for the presence of syphilis. Various technologies utilized include chemiluminescence immunoassay (CLIA), enzyme-linked immunosorbent assay (ELISA), among others. These diagnostics cater to applications in both men and women and serve diverse end-users such as hospitals, blood banks, diagnostic laboratories, and others.
The syphilis immunoassay diagnostics market research report is one of a series of new reports that provides syphilis immunoassay diagnostics market statistics, including the syphilis immunoassay diagnostics industry global market size, regional shares, competitors with syphilis immunoassay diagnostics market share, detailed syphilis immunoassay diagnostics market segments, market trends, and opportunities, and any further data you may need to thrive in the syphilis immunoassay diagnostics industry. These syphilis immunoassay diagnostics market research reports deliver a complete perspective of everything you need, with an in-depth analysis of the current and future scenarios of the industry.
The syphilis immunoassay diagnostics market consists of revenues earned by entities by providing services such as sample collection services, laboratory testing services, point-of-care testing services, and consultation and follow-up services. The market value includes the value of related goods sold by the service provider or included within the service offering. The syphilis immunoassay diagnostics market also consists of sales of rapid plasma reagin (RPR) tests, fluorescent treponemal antibody absorption (FTA-ABS) tests, point-of-care (POC) tests, western blot assays, and multiplex immunoassays. Values in this market are ‘factory gate’ values, that is the value of goods sold by the manufacturers or creators of the goods, whether to other entities (including downstream manufacturers, wholesalers, distributors and retailers) or directly to end customers. The value of goods in this market includes related services sold by the creators of the goods.
The market value is defined as the revenues that enterprises gain from the sale of goods and/or services within the specified market and geography through sales, grants, or donations in terms of the currency (in USD, unless otherwise specified).
The revenues for a specified geography are consumption values that are revenues generated by organizations in the specified geography within the market, irrespective of where they are produced. It does not include revenues from resales along the supply chain, either further along the supply chain or as part of other products.
This product will be delivered within 3-5 business days.
Table of Contents
Executive Summary
Syphilis Immunoassay Diagnostics Global Market Report 2025 provides strategists, marketers and senior management with the critical information they need to assess the market.This report focuses on syphilis immunoassay diagnostics market which is experiencing strong growth. The report gives a guide to the trends which will be shaping the market over the next ten years and beyond.
Reasons to Purchase:
- Gain a truly global perspective with the most comprehensive report available on this market covering 15 geographies.
- Assess the impact of key macro factors such as conflict, pandemic and recovery, inflation and interest rate environment and the 2nd Trump presidency.
- Create regional and country strategies on the basis of local data and analysis.
- Identify growth segments for investment.
- Outperform competitors using forecast data and the drivers and trends shaping the market.
- Understand customers based on the latest market shares.
- Benchmark performance against key competitors.
- Suitable for supporting your internal and external presentations with reliable high quality data and analysis
- Report will be updated with the latest data and delivered to you along with an Excel data sheet for easy data extraction and analysis.
- All data from the report will also be delivered in an excel dashboard format.
Description
Where is the largest and fastest growing market for syphilis immunoassay diagnostics ? How does the market relate to the overall economy, demography and other similar markets? What forces will shape the market going forward? The syphilis immunoassay diagnostics market global report answers all these questions and many more.The report covers market characteristics, size and growth, segmentation, regional and country breakdowns, competitive landscape, market shares, trends and strategies for this market. It traces the market’s historic and forecast market growth by geography.
- The market characteristics section of the report defines and explains the market.
- The market size section gives the market size ($b) covering both the historic growth of the market, and forecasting its development.
- The forecasts are made after considering the major factors currently impacting the market. These include: the Russia-Ukraine war, rising inflation, higher interest rates, and the legacy of the COVID-19 pandemic.
- Market segmentations break down the market into sub markets.
- The regional and country breakdowns section gives an analysis of the market in each geography and the size of the market by geography and compares their historic and forecast growth. It covers the growth trajectory of COVID-19 for all regions, key developed countries and major emerging markets.
- The competitive landscape chapter gives a description of the competitive nature of the market, market shares, and a description of the leading companies. Key financial deals which have shaped the market in recent years are identified.
- The trends and strategies section analyses the shape of the market as it emerges from the crisis and suggests how companies can grow as the market recovers.
Scope
Markets Covered:
1) By Product: Analyzers; Reagents; Kits; Other Products2) By Technology: Chemiluminescence Immunoassay (CLIA); Enzyme-Linked Immunosorbent Assay (ELISA); Other Technologies
3) By Application: Men; Women
4) By End-User: Hospitals; Blood Banks; Diagnostics Labs; Other End-Users
Subsegments:
1) By Analyzers: Automated Analyzers; Semi-Automated Analyzers; Manual Analyzers2) By Reagents: Enzyme-Linked Immunosorbent Assay Reagents; Chemiluminescent Immunoassay Reagents; Rapid Plasma Reagin Reagents; Treponemal and Non-Treponemal Reagents
3) By Kits: ELISA Kits; Rapid Diagnostic Test Kits; CLIA Kits; Point-of-Care Test Kits
4) By Other Products: Control and Calibration Materials; Sample Collection Devices; Ancillary Testing Accessories
Key Companies Mentioned: F. Hoffmann-La Roche Ltd.; Thermo Fisher Scientific Inc.; Abbott Laboratories; Siemens Healthineers; Becton Dickinson and Company
Countries: Australia; Brazil; China; France; Germany; India; Indonesia; Japan; Russia; South Korea; UK; USA; Canada; Italy; Spain
Regions: Asia-Pacific; Western Europe; Eastern Europe; North America; South America; Middle East; Africa
Time Series: Five years historic and ten years forecast.
Data: Ratios of market size and growth to related markets, GDP proportions, expenditure per capita.
Data Segmentation: Country and regional historic and forecast data, market share of competitors, market segments.
Sourcing and Referencing: Data and analysis throughout the report is sourced using end notes.
Delivery Format: PDF, Word and Excel Data Dashboard.
Companies Mentioned
The major companies featured in this Syphilis Immunoassay Diagnostics market report include:- F. Hoffmann-La Roche Ltd.
- Thermo Fisher Scientific Inc.
- Abbott Laboratories
- Siemens Healthineers
- Becton Dickinson and Company
- bioMérieux SA
- Bio-Rad Laboratories Inc.
- Meril Life Sciences Pvt. Ltd.
- Randox Laboratories Ltd.
- SEKISUI Diagnostics
- Bloodworks Northwest
- LetsGetChecked
- Everlywell Inc.
- ACON Laboratories Inc.
- Trinity Biotech
- Fujirebio
- CTK Biotech Inc.
- bioLytical Laboratories Inc.
- AdvaCare Pharma
- MyBioSource.com
- Gold Standard Diagnostics Frankfurt GmbH
- Newmarket Biomedical Ltd
- InTec PRODUCTS INC.
- NuGenerex Diagnostics LLC
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 200 |
| Published | April 2025 |
| Forecast Period | 2025 - 2029 |
| Estimated Market Value ( USD | $ 33.76 Billion |
| Forecasted Market Value ( USD | $ 45.42 Billion |
| Compound Annual Growth Rate | 7.7% |
| Regions Covered | Global |
| No. of Companies Mentioned | 25 |