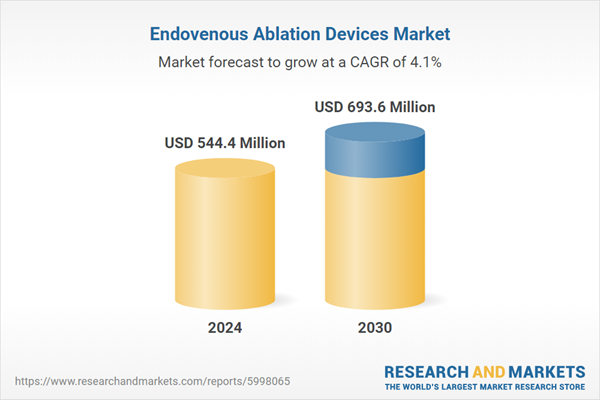
Global Endovenous Ablation Devices Market - Key Trends and Drivers Summarized
How Do Endovenous Ablation Devices Transform the Treatment of Varicose Veins?
Endovenous ablation devices have revolutionized the treatment of varicose veins, offering a minimally invasive alternative to traditional surgical methods like vein stripping. Varicose veins, characterized by swollen, twisted veins that often appear on the legs, result from faulty valves within the veins that cause blood to pool. This condition can lead to discomfort, pain, and more severe complications if left untreated. Endovenous ablation works by delivering thermal or chemical energy directly into the affected vein, causing it to collapse and seal shut. Over time, the treated vein is absorbed by the body, and blood flow is naturally redirected to healthier veins. This procedure, typically performed under local anesthesia, offers numerous advantages, including shorter recovery times, reduced pain, and minimal scarring compared to traditional surgery. The growing preference for endovenous ablation among both patients and healthcare providers reflects its effectiveness in providing long-term relief from varicose veins, making it the standard of care in many parts of the world.What Technological Innovations Are Enhancing Endovenous Ablation Devices?
Technological innovations are continually enhancing the safety, efficacy, and precision of endovenous ablation devices, broadening their application and improving patient outcomes. Radiofrequency ablation (RFA) and endovenous laser therapy (EVLT) are the two primary technologies used in these devices, each offering unique benefits. RFA uses radiofrequency energy to heat the vein wall, causing it to collapse, while EVLT employs laser energy to achieve the same result. Recent advancements in these technologies include the development of more compact, user-friendly devices with enhanced control features, allowing for greater precision during the procedure. Furthermore, innovations in catheter design, such as the introduction of closure fast catheters in RFA and radial fiber catheters in EVLT, have improved energy distribution within the vein, leading to more consistent results and reduced risk of complications. Another significant development is the advent of non-thermal, non-tumescent ablation methods, such as mechanochemical ablation (MOCA) and cyanoacrylate glue occlusion, which eliminate the need for heat and large volumes of anesthetic, further minimizing patient discomfort and recovery time. These technological advancements are making endovenous ablation procedures more accessible and effective, catering to a broader range of patient needs.How Are Patient Preferences and Healthcare Trends Influencing the Use of Endovenous Ablation Devices?
Patient preferences and broader healthcare trends are playing a crucial role in shaping the adoption of endovenous ablation devices. As patients increasingly seek treatments that are less invasive and require minimal downtime, the demand for endovenous ablation has surged. This shift is partly driven by the rise in outpatient procedures and the growing emphasis on value-based care, where the focus is on achieving the best possible outcomes at the lowest cost. The convenience of endovenous ablation, which often allows patients to return to normal activities within a day, aligns well with these trends. Additionally, the aesthetic benefits of the procedure, including the absence of large scars and the reduced risk of recurrence, have made it a popular choice among individuals concerned about the cosmetic aspects of varicose veins. The expanding demographic of aging populations, who are more prone to chronic venous insufficiency, further drives the demand for effective and convenient treatments like endovenous ablation. As healthcare providers increasingly prioritize patient-centered care, the popularity and utilization of these devices are expected to continue growing.What Is Driving Growth in the Endovenous Ablation Devices Market?
The growth in the endovenous ablation devices market is driven by several factors closely linked to technological advancements, changing patient demographics, and evolving healthcare practices. The increasing prevalence of varicose veins, particularly among the aging population and individuals with risk factors such as obesity and prolonged standing, has led to a greater need for effective treatment options. Technological advancements, such as the development of more efficient and safer ablation devices, have expanded the scope of these treatments, making them accessible to a broader patient base. The rising awareness of minimally invasive treatment options and the associated benefits of quicker recovery and improved cosmetic outcomes are also significant drivers. Additionally, the shift towards outpatient procedures and the increasing adoption of value-based healthcare models, which emphasize cost-effectiveness and patient satisfaction, have boosted the demand for endovenous ablation devices. The global expansion of healthcare infrastructure, particularly in emerging markets, is further propelling the market as access to advanced medical technologies improves. These factors, combined with ongoing innovations in device technology and procedural techniques, are fueling the continued growth of the endovenous ablation devices market.Report Scope
The report analyzes the Endovenous Ablation Devices market, presented in terms of market value. The analysis covers the key segments and geographic regions outlined below.- Segments: Device Type (Endovenous Laser Ablation Therapy (EVLT) Devices, Radiofrequency Ablation (RFA) Devices, Non-Thermal Non-Tumescent (NTNT) Devices).
- Geographic Regions/Countries: World; USA; Canada; Japan; China; Europe; France; Germany; Italy; UK; Spain; Russia; Rest of Europe; Asia-Pacific; Australia; India; South Korea; Rest of Asia-Pacific; Latin America; Argentina; Brazil; Mexico; Rest of Latin America; Middle East; Iran; Israel; Saudi Arabia; UAE; Rest of Middle East; Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Endovenous Laser Ablation Therapy (EVLT) Devices segment, which is expected to reach US$486.0 Million by 2030 with a CAGR of a 4.5%. The Radiofrequency Ablation (RFA) Devices segment is also set to grow at 3.9% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $146.7 Million in 2024, and China, forecasted to grow at an impressive 7.8% CAGR to reach $148.8 Million by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Endovenous Ablation Devices Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Endovenous Ablation Devices Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Endovenous Ablation Devices Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as Alfresa Pharma Corporation, AngioDynamics, Inc., AtriCure, Inc., Biolitec AG, Canyon Medical Inc. and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 51 companies featured in this Endovenous Ablation Devices market report include:
- Alfresa Pharma Corporation
- AngioDynamics, Inc.
- AtriCure, Inc.
- Biolitec AG
- Canyon Medical Inc.
- Eufoton Srl
- F Care Systems
- LSO Medical
- Merit Medical Systems, Inc.
- Olympus Europa SE & Co. KG
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Alfresa Pharma Corporation
- AngioDynamics, Inc.
- AtriCure, Inc.
- Biolitec AG
- Canyon Medical Inc.
- Eufoton Srl
- F Care Systems
- LSO Medical
- Merit Medical Systems, Inc.
- Olympus Europa SE & Co. KG
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 192 |
| Published | January 2026 |
| Forecast Period | 2024 - 2030 |
| Estimated Market Value ( USD | $ 544.4 Million |
| Forecasted Market Value ( USD | $ 693.6 Million |
| Compound Annual Growth Rate | 4.1% |
| Regions Covered | Global |