Market Size & Trends
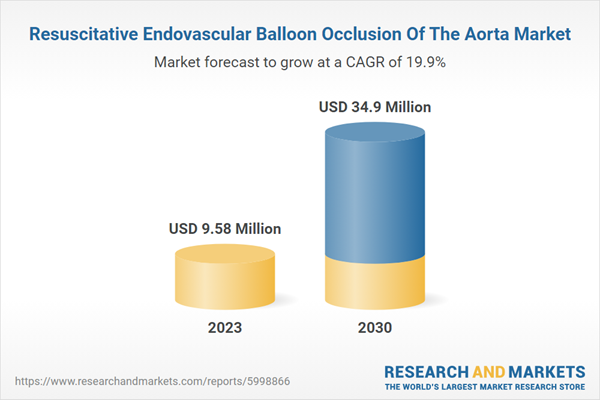
The global resuscitative endovascular balloon occlusion of the aorta market size was valued at USD 9.58 million in 2023 and is projected to grow at a CAGR of 19.9% from 2024 to 2030. The market is driven by several key factors that influence its growth and adoption in clinical settings. These drivers include the increasing prevalence of traumatic injuries, advancements in medical technology, a growing emphasis on trauma care protocols, rising awareness among healthcare professionals, and supportive government initiatives. Each of these elements plays a crucial role in shaping the market landscape and enhancing the utilization of REBOA as a life-saving intervention.The rising incidence of traumatic injuries, particularly those resulting from road accidents, falls, and violence, is a significant driver for the Resuscitative Endovascular Balloon Occlusion of the Aorta (REBOA) market. As trauma cases often lead to hemorrhagic shock, the demand for effective resuscitation techniques has surged. REBOA provides a minimally invasive option that can be rapidly deployed in emergency situations, making it an attractive choice for trauma surgeons and emergency medical services. In June 2024, the World Health Organization reported that injuries, both unintentional and violence-related, significantly contribute to trauma, leading to 4.4 million deaths annually. Exposure to trauma, especially during childhood, can heighten the risk of mental health issues, substance abuse, chronic diseases, and social problems.
Technological advancements in medical devices have significantly enhanced the efficacy and safety of REBOA procedures. Innovations such as improved balloon designs, better catheter systems, and enhanced imaging technologies facilitate more precise placement and monitoring during procedures. These advancements not only improve patient outcomes but also encourage healthcare providers to adopt REBOA as a standard practice in trauma care. In June 2024, Emergency Scientific, a Utah-based firm, announced the first use of its Landmark REBOA catheter, following its FDA 510(k) clearance. This device is for temporary occlusion of large vessels in emergency hemorrhage control. The Landmark catheter is designed to enhance the precision of occlusion while minimizing complications associated with traditional methods.
There is an increasing awareness among healthcare professionals regarding the benefits of REBOA in managing traumatic hemorrhage. Alongside this awareness, specialized training programs are being developed to educate clinicians on the proper use of REBOA techniques. This growing emphasis on education ensures that more healthcare providers are equipped with the knowledge and skills necessary to implement this life-saving procedure effectively. In March 2024, an article was published in the Journal of Emergency Medicine detailing A Pilot Study on A Novel REBOA Training Curriculum designed for Emergency Medicine Residents. This cutting-edge curriculum was designed to advance the competencies and understanding of Emergency Medicine residents in applying the REBOA technique, with the aim of bettering patient outcomes in life-threatening scenarios.
The regulatory environment surrounding medical devices has become increasingly supportive of innovative solutions like REBOA. Regulatory bodies are recognizing the importance of rapid intervention techniques in trauma care and are streamlining approval processes for new devices. This supportive stance encourages manufacturers to invest in research and development, further propelling market growth. In February 2024, Neurescue announced that it raised USD 7.27 million in a Series A funding round. This investment was spearheaded by West Hill Capital, a London-based investment firm.
Global Resuscitative Endovascular Balloon Occlusion Of The Aorta Market Report Segmentation
This report forecasts revenue growth at global, regional, and country levels and provides an analysis of the latest industry trends in each of the sub-segments from 2018 to 2030. For this study, Grand View Research has segmented the global resuscitative endovascular balloon occlusion of the aorta market report based on end-use, and region:- End-use Outlook (Revenue, USD Million, 2018 - 2030)
- Cardiac Arrest
- Aortic Occlusion
- Tactical Combat Casualty Care
- Regional Outlook (Revenue, USD Million, 2018 - 2030)
- North America
- U.S.
- Canada
- Europe
- Germany
- UK
- France
- Italy
- Spain
- Asia Pacific
- China
- Japan
Why should you buy this report?
- Comprehensive Market Analysis: Gain detailed insights into the global market across major regions and segments.
- Competitive Landscape: Explore the market presence of key players worldwide.
- Future Trends: Discover the pivotal trends and drivers shaping the future of the global market.
- Actionable Recommendations: Utilize insights to uncover new revenue streams and guide strategic business decisions.
This report addresses:
- Market intelligence to enable effective decision-making
- Market estimates and forecasts from 2018 to 2030
- Growth opportunities and trend analyses
- Segment and regional revenue forecasts for market assessment
- Competition strategy and market share analysis
- Product innovation listing for you to stay ahead of the curve
- COVID-19's impact and how to sustain in these fast-evolving markets
Table of Contents
Companies Mentioned
- Prytime Medical Devices, Inc.
- NEURESCUE
- Emergency Scientific, LLC
- Qx Médical
- Front Line Medical Technologies
- Reboa Medical As
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 120 |
| Published | August 2024 |
| Forecast Period | 2023 - 2030 |
| Estimated Market Value ( USD | $ 9.58 Million |
| Forecasted Market Value ( USD | $ 34.9 Million |
| Compound Annual Growth Rate | 19.9% |
| Regions Covered | Global |
| No. of Companies Mentioned | 6 |