The global cardiac prosthetic devices market demand is driven by the rising prevalence of valve disorders, heart failure, and congenital heart defects. Increasing demand for advanced treatment options has driven innovations in artificial heart valves, enhancing patient outcomes. Advancements in biocompatible materials have improved durability and reduced complications, making these devices more effective. Additionally, mechanical circulatory support devices are gaining traction as crucial solutions for severe cardiac conditions. The growing adoption of artificial heart valves in minimally invasive procedures further boosts market expansion. Moreover, mechanical circulatory support devices play a vital role in maintaining cardiac function in patients with end-stage heart disease. The global market is expected to grow steadily due to rising healthcare investments, improved surgical techniques, and increasing awareness of advanced cardiac treatments. As the burden of cardiovascular diseases rises, the demand for innovative prosthetic solutions continues to drive market progress, ensuring better patient care worldwide.
The rising aging global population and increasing burden of cardiovascular diseases (CVDs) are key factors driving the demand for cardiac prosthetic devices. With a growing focus on improving cardiovascular health, advancements in medical technology have led to innovative solutions for managing heart conditions. Continuous progress in surgical techniques has enhanced the efficiency of implanting cardiac prosthetic devices, reducing recovery times and improving patient outcomes. The expansion of treatment options such as transcatheter heart valves has further strengthened market growth. Investments in research and development (R&D) are fostering next-generation prosthetic innovations, offering better biocompatibility and longevity. Additionally, the healthcare sector is increasingly adopting personalised solutions, ensuring improved patient-centric care. The rising incidence of cardiovascular diseases worldwide underscores the urgent need for technologically advanced prosthetic solutions. With ongoing advancements, the market is set to witness sustained growth, addressing the evolving demands of healthcare providers and patients alike.
Cardiac Prosthetic Devices Market Trends/Drivers
Several trends and developments are being observed in the market to enhance the current situation. Some of the noteworthy trends are as follows:Rising Incidence of Cardiovascular Diseases and their Awareness Boosts Market Growth
The growing incidence of cardiovascular diseases, including coronary artery disease, heart failure, and valvular heart diseases, is significantly driving demand for cardiac prosthetic devices. With increasing awareness, early diagnosis and timely interventions are improving patient outcomes. Advancements in treatment options have led to innovations in cardiac prosthetics, enhancing durability and functionality. Additionally, advanced imaging techniques such as echocardiography and cardiac MRI are improving precision in diagnosing and treating complex cardiac conditions. Government initiatives, healthcare campaigns, and non-profit organisations are actively promoting heart health awareness, and encouraging regular screenings along with preventive measures. As the global burden of cardiovascular diseases continues to rise, the demand for technologically advanced and minimally invasive prosthetic solutions is increasing. These innovations are improving patient survival rates, reducing hospital stays, and enhancing the overall quality of life for individuals with severe heart conditions.Surge in Favorable Reimbursement Policies to Augment Market Value
The increasing implementation of reimbursement policies is significantly driving the cardiac prosthetic devices market. Governments and private insurers worldwide are expanding healthcare coverage, reducing out-of-pocket expenses for patients requiring advanced cardiac treatments. Enhanced reimbursement frameworks improve the affordability of cardiac prosthetic devices, making them more accessible to a larger patient population. These policies encourage hospitals and clinics to adopt cutting-edge medical technology, further propelling market growth. Additionally, improved healthcare funding fosters innovation in cardiac prosthetic devices, ensuring better treatment outcomes. Strengthened healthcare infrastructure and supportive regulations also enhance accessibility, allowing more patients to benefit from life-saving cardiac interventions. As governments prioritise healthcare, the demand for cardiac prosthetic devices is expected to rise steadily. Expanding insurance coverage and favourable pricing strategies will continue to shape the market, ensuring broader adoption and increased patient access to advanced cardiac solutions.Surge in Technological Innovations to Improve Cardiac Surgery Techniques and Devices
Advancements in cardiac surgical techniques are transforming patient care, with increasing adoption of minimally invasive procedures that reduce complications and recovery time. Cutting-edge robotic-assisted surgeries and transcatheter interventions are enhancing procedural accuracy and patient outcomes. The demand for durable cardiac prosthetic devices is rising, particularly for transcatheter heart valves, which improve success rates in valve replacement procedures. Additionally, innovations in ventricular assist devices (VADs) and pacemakers integrate wireless connectivity and remote monitoring, ensuring proactive patient management. Furthermore, advanced sensors optimise real-time data collection, enhancing diagnostics and device efficiency. As technology progresses, next-generation heart valves with superior biocompatibility and longevity are being developed. These innovations are reshaping the market, offering safer and more effective solutions for patients with cardiovascular conditions. Ongoing research and investments in cutting-edge technology will continue to drive market growth and improve cardiac care worldwide.Global Cardiac Prosthetic Devices Industry Segmentation
This report provides an analysis of forecasts for revenue growth at the global, regional, and country levels, and the latest industry trends in each of the sub-segments from 2025 to 2034. For this study, Expert Market Research has segmented the global cardiac prosthetic devices market report based on product type, end-user, and region.Market Breakup by Product Type
- Valves
- Tissue Valve
- Transcatheter Valve
- Mechanical Valve
- Others
- Pacemakers
- Implantable Pacemakers
- External Pacemaker
- Others
Market Breakup by End User
- Hospitals & Clinics
- Ambulatory Surgical Centers (ASCs)
- Others
Market Breakup by Region
- North America
- Europe
- Asia Pacific
- Latin America
- Middle East and Africa
Valves dominate the market
The market for cardiac prosthetic devices is segmented by product type, including valves and pacemakers. Valves dominate the sector, encompassing mechanical valves, tissue valves, stented tissue valves, stentless tissue valves, and transcatheter valves. Their widespread adoption is due to enhanced durability and biocompatibility. Meanwhile, pacemakers, comprising implantable and external pacemakers, are gaining traction due to rising cardiovascular conditions. Implantable pacemakers include single-chamber, dual-chamber, and triple-chamber battery pacemakers, offering tailored cardiac rhythm management. Innovations in valves and pacemakers continue to drive industry growth, improving patient outcomes and treatment efficiency.Advancements in valve design are enhancing durability and function, leading to broader adoption of valve products. The shift toward minimally invasive procedures is increasing demand for transcatheter heart valve replacement, particularly for high-risk patients. Improved materials and next-generation solutions are enhancing longevity and biocompatibility, reducing complications. Ongoing research is expected to drive further innovations in valve products worldwide.
Developments in medical technology are driving market growth, with innovative pacemaker devices integrating wireless connectivity and remote monitoring capabilities. The rising prevalence of lifestyle-related diseases, including obesity, diabetes, and related heart ailments, is increasing demand for pacemakers. These advancements improve patient care by enabling real-time monitoring and early detection of complications, ensuring better clinical outcomes.
Hospitals, clinics and cardiac centers hold the largest share in the market
The market is segmented by end-user, including hospitals, clinics, cardiac centers, and ambulatory surgical centers. Hospitals, clinics, and cardiac centers dominate due to advanced medical facilities and skilled specialists. However, ambulatory surgical centers are gaining traction due to cost-effective, efficient treatments. Their ability to perform cardiac procedures with shorter recovery times contributes to increasing demand in the sector.The rising adoption of innovative devices like artificial heart valves and implantable pacemakers in hospitals, clinics, and cardiac centers is enhancing cardiovascular care. Continuous advancements in medical technology and surgical techniques allow these facilities to provide cutting-edge treatment options. Moreover, government initiatives and healthcare reforms focus on improving patient outcomes, further driving demand. These factors make hospitals, clinics, and cardiac centers pivotal in expanding the market for cardiac prosthetic devices.
Ambulatory surgical centers (ASCs) play a crucial role in performing cardiac procedures, offering cost-effective and efficient solutions. With advancements in minimally invasive surgeries, ASCs provide safe outpatient treatments, reducing hospital stays. The shift towards value-based healthcare has increased patient preference for ASCs. Improved technology now enables complex cardiac procedures in these settings, further boosting market demand.
North America exhibits a clear dominance, accounting for the largest cardiac prosthetic devices market share
The global cardiac prosthetic devices market is expanding across key regional markets, including North America, Asia Pacific, Europe, Latin America, and the Middle East and Africa. North America leads due to advanced healthcare facilities and high adoption of cutting-edge technologies. Europe follows, benefiting from strong research initiatives and increasing cardiac surgeries. The Asia Pacific region is witnessing rapid growth due to rising healthcare investments and an expanding patient pool. Meanwhile, Latin America along with the Middle East and Africa are experiencing steady growth, driven by improving healthcare infrastructure and increasing awareness of cardiovascular health.
North America dominates the market due to the high prevalence of cardiovascular diseases and the rising demand for advanced heart valves, pacemakers, and implantable defibrillators. The region’s robust healthcare system supports innovation, while strong reimbursement support ensures patient access to life-saving treatments. Additionally, significant research and development efforts drive the creation of innovative and minimally invasive prosthetic devices, improving patient outcomes. Increasing investments in medical technology and favourable regulatory frameworks further enhance market expansion. With ongoing advancements and a growing patient base, North America remains a key hub for cardiac prosthetic device development and adoption.
Asia Pacific is experiencing rapid growth due to its expanding aging population and changing lifestyles, which are increasing the prevalence of cardiovascular conditions. Improvements in healthcare infrastructure and rising disposable income have enhanced access to medical treatments, improving overall patient care. However, affordability remains a key concern, influencing market penetration. Ongoing technological advancements and innovations in cardiac prosthetic devices are further accelerating market expansion. As awareness and accessibility continue to rise, the Asia Pacific region is expected to play a crucial role in shaping the future of the global cardiac prosthetic devices market.
Competitive Landscape
The market for cardiac prosthetic devices is becoming increasingly dynamic, driven by advancements in medical technology and evolving surgical procedures. Companies are investing heavily in research and development to create innovative devices that enhance patient outcomes. The integration of digital health technologies and remote monitoring solutions is transforming post-surgical care, enabling real-time health tracking. Furthermore, 3D printing technology is revolutionising the sector by facilitating the production of patient-specific devices, offering customizable solutions for improved compatibility. These advancements, along with personalized healthcare services, are shaping the future of cardiac prosthetics, ensuring efficient devices tailored to individual patient needs. Additionally, the growing focus on tailored solutions allows healthcare professionals to deliver more precise and effective treatments, improving overall cardiac care.The competitive landscape of the market is characterised by continuous innovation and strategic growth initiatives. Key players are strengthening their market presence through collaborations, acquisitions, and product development, with an emphasis on data-driven analysis and technological advancements. Some of the key players in the market include:
- Abbott Laboratories
- LivaNova PLC
- Medtronic Plc
- Boston Scientific Corporation
- Edwards Lifesciences Corporation
- Colibri Heart Valve
- Meril Life Sciences Pvt Ltd
- BioTronik
- Lepu Medical Technology Co Ltd
- Siemens Healthineers
- Sorin Group
- St. Jude Medical Inc
Recent Developments
In July 2024, Octagos Health secured USD 43 million in Series B funding, led by Morgan Stanley Expansion Capital. The investment will enhance its AI-driven platform for continuous monitoring of pacemakers, defibrillators, and wearable cardiac devices, improving real-time data analysis and remote patient management in advanced cardiac care.In June 2024, Medtronic plc launched the Avalus Ultra valve, a next-generation surgical aortic tissue valve designed for enhanced ease of implantation and lifetime patient management. This innovation aims to provide a durable and future-ready aortic valve solution for cardiac surgeons and patients. The Avalus Ultra valve enhances surgical outcomes, supporting the growing demand for advanced cardiac prosthetic devices in valve replacement procedures.
In May 2024, the FDA approved the Edwards SAPIEN 3, SAPIEN 3 Ultra, and SAPIEN 3 Ultra RESILIA Transcatheter Heart Valve System for replacing failing surgical biological mitral valves in patients at intermediate or high risk for open-heart surgery. The system, featuring a catheter-based artificial heart valve, was previously approved for treating aortic stenosis and failing surgical or transcatheter aortic and mitral valves in high-risk patients.
In February 2024, the FDA approved the Edwards EVOQUE Tricuspid Valve Replacement System, featuring a bioprosthetic tricuspid valve and delivery catheter. Made from cow tissue and a self-expanding metal frame, it enables minimally invasive valve replacement, preventing blood backflow into the right atrium without requiring open-heart surgery, and improving patient outcomes.
A WHO/Europe report highlighted that hypertension is commonly caused by excessive salt consumption and affects around 1 in 3 adults (aged between 30-79) in the European Region. CVDs cause 42.5% of deaths annually, with men 2.5 times more likely to die than women. The report urges salt reduction and better hypertension control.
This product will be delivered within 3-5 business days.
Table of Contents
Companies Mentioned
- Abbott
- Medtronic plc
- Boston Scientific Corporation
- Edwards Lifesciences Corporation
- Biotronik
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 400 |
| Published | June 2025 |
| Forecast Period | 2025 - 2034 |
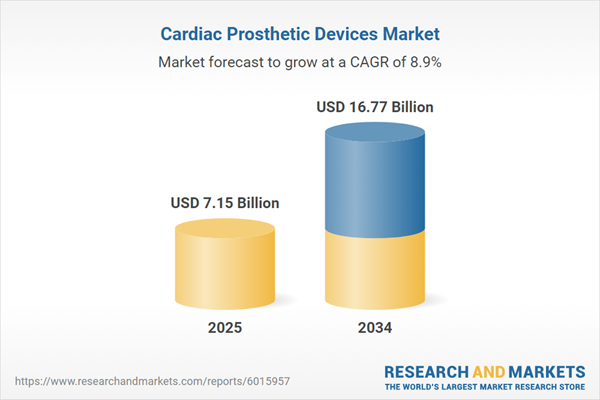
| Estimated Market Value ( USD | $ 7.15 Billion |
| Forecasted Market Value ( USD | $ 16.77 Billion |
| Compound Annual Growth Rate | 8.9% |
| Regions Covered | Global |
| No. of Companies Mentioned | 5 |