Global Point-of-Care Infectious Disease Diagnostics Market - Key Trends & Drivers Summarized
Why Are Point-of-Care Infectious Disease Diagnostics Transforming Healthcare Delivery and Patient Outcomes?
Point-of-care (POC) infectious disease diagnostics are revolutionizing healthcare by enabling rapid, accurate, and decentralized testing of infectious diseases at or near the site of patient care. These diagnostics include a range of tests, such as lateral flow assays, rapid antigen tests, molecular diagnostic assays, and immunoassays, which can be performed outside traditional laboratory settings, providing healthcare providers with critical information for timely clinical decision-making. Unlike conventional diagnostic methods that require centralized laboratories, specialized equipment, and longer turnaround times, POC diagnostics deliver results within minutes to hours, allowing for immediate diagnosis and initiation of treatment. This rapid testing capability is particularly vital in the management of infectious diseases such as HIV, tuberculosis, influenza, and sexually transmitted infections (STIs), where early detection and prompt treatment are crucial to controlling disease spread and improving patient outcomes.The growing adoption of POC infectious disease diagnostics is driven by their ability to increase access to testing, particularly in remote, resource-limited, and underserved areas. These diagnostics eliminate the need for complex laboratory infrastructure, making it possible to perform testing in rural clinics, community health centers, and even in patients' homes. This accessibility is crucial for addressing global health challenges, especially in low - and middle-income countries where healthcare resources are limited and the burden of infectious diseases is high. Moreover, the convenience and ease of use of POC diagnostics enhance patient compliance and reduce the need for follow-up visits, supporting more efficient healthcare delivery and reducing the burden on healthcare systems. As the demand for rapid and decentralized testing continues to grow, POC infectious disease diagnostics are poised to play an increasingly important role in global health, enabling better disease management, faster outbreak response, and more effective control of infectious diseases.
What Technological Advancements Are Driving the Evolution and Adoption of Point-of-Care Infectious Disease Diagnostics?
Technological advancements are significantly enhancing the accuracy, speed, and versatility of point-of-care infectious disease diagnostics, making them more effective and widely applicable. One of the most transformative innovations in this field is the development of molecular POC diagnostics, which utilize techniques such as polymerase chain reaction (PCR) and isothermal amplification to detect pathogens at the genetic level. Molecular diagnostics offer high sensitivity and specificity, enabling the detection of even low levels of pathogens in patient samples. The miniaturization of molecular diagnostic platforms and the integration of lab-on-a-chip technologies are making it possible to perform complex molecular tests at the point of care, providing results that are comparable to those obtained in centralized laboratories. These advancements are expanding the use of molecular POC diagnostics for detecting a wide range of pathogens, including bacteria, viruses, and fungi, and are enabling multiplex testing to identify multiple infections simultaneously from a single sample.Another significant technological advancement is the development of rapid antigen and antibody tests, which provide immediate results for detecting active infections or prior exposure to pathogens. Lateral flow assays (LFAs), a common type of rapid antigen test, are widely used for detecting infectious diseases such as COVID-19, influenza, malaria, and HIV. These tests are designed to be user-friendly, portable, and capable of delivering results within minutes, making them ideal for use in both clinical and non-clinical settings. Recent innovations in lateral flow technology, such as enhanced signal detection and the use of nanoparticle labels, are improving the sensitivity and reliability of these tests, supporting their broader adoption in disease surveillance and community screening programs. Additionally, the development of next-generation lateral flow assays with smartphone-based readout systems is enabling digital analysis and real-time data sharing, enhancing the integration of POC diagnostics into digital health platforms.
The integration of digital technologies and artificial intelligence (AI) is further driving the evolution of point-of-care infectious disease diagnostics. Digital POC diagnostics are being developed with connectivity features that allow them to transmit test results to electronic health records (EHRs), cloud-based platforms, or mobile apps, enabling remote monitoring and telehealth consultations. The use of AI and machine learning algorithms is enhancing the interpretation of test results, reducing the potential for human error, and providing actionable insights for clinicians. AI-driven POC diagnostics are also being used to develop predictive models for disease outbreaks and to optimize diagnostic workflows in field settings. The combination of digital technologies and AI is enabling more efficient data collection, analysis, and reporting, making POC diagnostics powerful tools for public health monitoring and epidemiological research. As technology continues to advance, POC infectious disease diagnostics are expected to become even more accurate, accessible, and integrated with broader healthcare systems, supporting more effective disease management and control.
How Are Market Dynamics and Regulatory Standards Shaping the Point-of-Care Infectious Disease Diagnostics Market?
The point-of-care infectious disease diagnostics market is shaped by a complex interplay of market dynamics, regulatory standards, and healthcare trends that are influencing product development, adoption, and commercialization. One of the primary market drivers is the increasing prevalence of infectious diseases, both in developed and developing regions, which is creating a growing demand for rapid and accessible testing solutions. Infectious diseases such as HIV, tuberculosis, hepatitis, and malaria continue to pose significant health challenges globally, particularly in low - and middle-income countries where diagnostic infrastructure is limited. The recent COVID-19 pandemic has further underscored the need for rapid and widespread testing, highlighting the critical role of POC diagnostics in outbreak response and public health management. As healthcare systems around the world strive to improve infectious disease detection and control, the demand for POC diagnostics that can deliver reliable results quickly and efficiently is expected to rise.Regulatory standards and compliance requirements are also playing a crucial role in shaping the point-of-care infectious disease diagnostics market. Regulatory agencies such as the U.S. Food and Drug Administration (FDA), the European Medicines Agency (EMA), and other regional bodies have established stringent guidelines for the development, approval, and commercialization of diagnostic tests to ensure their safety, efficacy, and reliability. Compliance with these regulations is essential for manufacturers to gain market access and maintain the trust of healthcare providers and patients. The regulatory landscape is evolving to accommodate the rapid development and deployment of POC diagnostics, particularly in response to public health emergencies. For example, the FDA's Emergency Use Authorization (EUA) process, which was widely used during the COVID-19 pandemic, facilitated the expedited approval of rapid tests for SARS-CoV-2, enabling their rapid distribution and use in healthcare and community settings. Similar regulatory pathways are being explored in other regions to support the development of POC diagnostics for emerging infectious diseases.
Market dynamics such as competition among manufacturers, technological innovation, and healthcare reimbursement policies are also influencing the point-of-care infectious disease diagnostics market. The competitive landscape is characterized by the presence of established diagnostic companies and innovative startups, each striving to develop novel POC testing solutions that offer superior performance, ease of use, and affordability. Companies are differentiating themselves through product innovation, strategic partnerships, and the ability to provide comprehensive diagnostic solutions that include devices, reagents, and digital health integrations. Pricing pressures, particularly in cost-sensitive markets, are driving the demand for low-cost POC tests that offer high performance without compromising quality. Additionally, healthcare reimbursement policies and coverage decisions by payers are impacting the adoption of POC diagnostics, as reimbursement often determines the accessibility and affordability of these products. Reimbursement environments vary significantly across regions and are influenced by factors such as the clinical utility of the test, the availability of alternative diagnostic options, and the overall healthcare budget. Navigating these market dynamics and regulatory standards is essential for companies operating in the point-of-care infectious disease diagnostics market as they seek to expand their presence and address the unmet diagnostic needs of patients and healthcare providers worldwide.
What Are the Key Growth Drivers Fueling the Expansion of the Point-of-Care Infectious Disease Diagnostics Market?
The growth in the global point-of-care infectious disease diagnostics market is driven by several key factors, including the increasing prevalence of infectious diseases, the growing demand for rapid and decentralized testing solutions, and advancements in diagnostic technologies. One of the primary growth drivers is the rising incidence of infectious diseases worldwide, which is creating a significant need for diagnostic tools that can provide early detection and support timely treatment decisions. Infectious diseases such as HIV, tuberculosis, hepatitis, and sexually transmitted infections (STIs) continue to be major public health challenges, particularly in low - and middle-income countries. Point-of-care diagnostics are playing a critical role in addressing these challenges by enabling rapid and accurate testing in decentralized settings, such as rural clinics, community health centers, and home care environments. The ability of POC diagnostics to deliver results quickly and at the site of care is enhancing patient access to testing, supporting early diagnosis, and enabling immediate initiation of treatment, which is crucial for improving patient outcomes and reducing disease transmission.Another significant growth driver is the increasing focus on rapid and decentralized testing solutions, particularly in the context of global health emergencies such as the COVID-19 pandemic. The pandemic has highlighted the importance of scalable and accessible diagnostic solutions for controlling disease spread and managing public health. POC diagnostics, with their ability to provide real-time results without the need for complex laboratory infrastructure, have become essential tools in the fight against COVID-19 and other emerging infectious diseases. The experience gained during the pandemic is expected to drive the continued adoption of POC diagnostics for other infectious diseases, as healthcare systems seek to build more resilient and responsive diagnostic capabilities. The growing demand for at-home and self-testing options is also contributing to market growth, as these tests offer convenience and support disease management in non-traditional healthcare settings.
Advancements in diagnostic technologies are further fueling the expansion of the point-of-care infectious disease diagnostics market. Innovations such as molecular POC platforms, digital diagnostics, and connectivity features are enabling the development of tests that offer higher sensitivity, specificity, and ease of use. Molecular POC platforms, which utilize techniques such as PCR and isothermal amplification, are providing highly accurate results for detecting a wide range of pathogens, from bacteria and viruses to fungi. Digital diagnostics with smartphone-based readout systems and cloud connectivity are enabling remote monitoring and integration with telehealth platforms, supporting more comprehensive disease management and surveillance. The development of multiplex POC tests, which can detect multiple pathogens simultaneously from a single sample, is also expanding the capabilities of POC diagnostics, making them more versatile and efficient.
Lastly, the increasing focus on public health initiatives and the expansion of diagnostic infrastructure in emerging markets are contributing to the growth of the point-of-care infectious disease diagnostics market. In regions such as Asia-Pacific, Africa, and Latin America, where the burden of infectious diseases is high and access to laboratory testing is limited, POC diagnostics are providing a solution for improving disease detection and control. Governments, non-profit organizations, and global health agencies are investing in diagnostic infrastructure and community-based testing programs to enhance access to care and reduce disease burden. The increasing availability of affordable POC diagnostics in these regions is supporting the broader adoption of testing and improving health outcomes. As demand from key sectors such as public health, primary care, and home healthcare continues to rise, and as manufacturers innovate to meet evolving healthcare needs, the global point-of-care infectious disease diagnostics market is expected to witness sustained growth, driven by advancements in technology, expanding applications, and the increasing emphasis on rapid and decentralized testing solutions.
Report Scope
The report analyzes the Point-of-Care Infectious Disease Diagnostics market, presented in terms of market value (US$ Thousand). The analysis covers the key segments and geographic regions outlined below.- Segments: Technique (Lateral Flow Immunoassay, Flow-through Test, Molecular Diagnostics, Agglutination Test, Other Techniques); End-Use (Clinics End-Use, Hospitals End-Use, Home End-Use, Assisted Living Healthcare Facilities End-Use, Other End-Uses).
- Geographic Regions/Countries:World; USA; Canada; Japan; China; Europe; France; Germany; Italy; UK; Spain; Russia; Rest of Europe; Asia-Pacific; Australia; India; South Korea; Rest of Asia-Pacific; Latin America; Argentina; Brazil; Mexico; Rest of Latin America; Middle East; Iran; Israel; Saudi Arabia; UAE; Rest of Middle East; Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Lateral Flow Immunoassay Technique segment, which is expected to reach US$2.7 Billion by 2030 with a CAGR of a 11.5%. The Flow-through Test Technique segment is also set to grow at 10.5% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $786.2 Million in 2024, and China, forecasted to grow at an impressive 14.7% CAGR to reach $1.2 Billion by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Point-of-Care Infectious Disease Diagnostics Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Point-of-Care Infectious Disease Diagnostics Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Point-of-Care Infectious Disease Diagnostics Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as AccuBioTech Co., Ltd., Agilent Technologies, Inc., BioFire Diagnostics LLC, bioMerieux SA, Bio-Rad Laboratories, Inc. and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 38 companies featured in this Point-of-Care Infectious Disease Diagnostics market report include:
- AccuBioTech Co., Ltd.
- Agilent Technologies, Inc.
- BioFire Diagnostics LLC
- bioMerieux SA
- Bio-Rad Laboratories, Inc.
- Cardinal Health, Inc.
- Cepheid
- Chembio Diagnostic Systems, Inc.
- Danaher Corporation
- EKF Diagnostics Holdings PLC
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- AccuBioTech Co., Ltd.
- Agilent Technologies, Inc.
- BioFire Diagnostics LLC
- bioMerieux SA
- Bio-Rad Laboratories, Inc.
- Cardinal Health, Inc.
- Cepheid
- Chembio Diagnostic Systems, Inc.
- Danaher Corporation
- EKF Diagnostics Holdings PLC
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 287 |
| Published | February 2026 |
| Forecast Period | 2024 - 2030 |
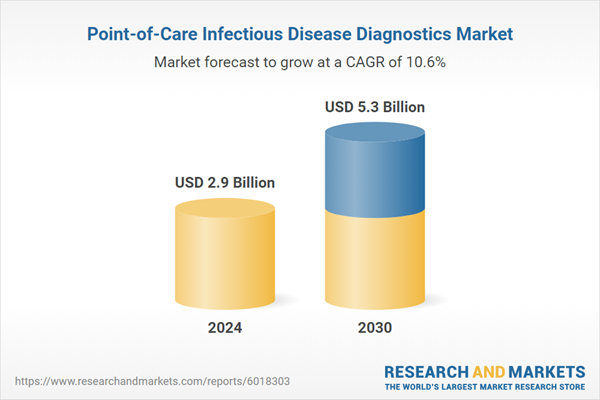
| Estimated Market Value ( USD | $ 2.9 Billion |
| Forecasted Market Value ( USD | $ 5.3 Billion |
| Compound Annual Growth Rate | 10.6% |
| Regions Covered | Global |