Global Nephrostomy Devices Market - Key Trends & Drivers Summarized
Why Are Nephrostomy Devices Essential in Urological Interventions?
Nephrostomy devices play a crucial role in urological medicine, particularly in cases where urine flow is obstructed due to conditions such as kidney stones, tumors, or severe infections. These devices provide an artificial drainage route by inserting a catheter into the renal pelvis through the skin, allowing urine to bypass blockages and preventing kidney damage. Nephrostomy procedures are typically performed when ureteral stenting is not feasible or effective, making them a vital option for patients experiencing severe hydronephrosis or post-surgical complications. Technological advancements have led to the development of high-quality nephrostomy catheters that are more durable, biocompatible, and resistant to infections. The increasing prevalence of kidney-related disorders, particularly in aging populations and individuals with comorbidities such as diabetes and hypertension, has driven the demand for nephrostomy devices. Additionally, the growing adoption of minimally invasive surgical techniques has enhanced the precision and safety of nephrostomy placements, reducing recovery times and improving patient outcomes. As medical advancements continue to optimize nephrostomy procedures, these devices remain essential for managing complex urological conditions effectively.What Challenges Are Impacting the Nephrostomy Devices Market?
Despite the critical role of nephrostomy devices, the market faces several challenges that impact their adoption and accessibility. One of the primary concerns is the risk of infections and complications associated with long-term nephrostomy tube placement. Catheter-related infections, leakage, and accidental dislodgment can lead to severe complications, necessitating frequent monitoring and device replacements. Additionally, patient discomfort and the need for regular catheter care can limit patient compliance and overall satisfaction. Another significant challenge is the lack of trained healthcare professionals in certain regions, particularly in developing countries, where nephrostomy procedures are less common due to limited access to advanced urological care. Furthermore, reimbursement policies and the high cost of nephrostomy devices can restrict their availability, particularly in low-resource healthcare settings. The variability in regulatory requirements across different regions also poses hurdles for manufacturers seeking market expansion. Addressing these challenges requires advancements in device design to reduce infection risks, improved training programs for healthcare providers, and policies that enhance accessibility and affordability of nephrostomy procedures worldwide.How Are Innovations in Nephrostomy Devices Enhancing Patient Outcomes?
Technological advancements are significantly improving the safety, efficacy, and patient experience of nephrostomy procedures. The development of antimicrobial-coated catheters is helping to reduce the risk of infections, which has been a persistent challenge in nephrostomy care. Additionally, bioengineered materials are being incorporated into catheter designs to enhance durability and biocompatibility, minimizing tissue irritation and improving long-term outcomes. Another major innovation is the integration of real-time imaging technologies, such as fluoroscopy and ultrasound guidance, which allows for more precise catheter placement and reduces complications during insertion. The rise of robotic-assisted nephrostomy procedures is further improving accuracy while minimizing trauma to surrounding tissues. Smart nephrostomy devices equipped with sensors and remote monitoring capabilities are also being explored, allowing healthcare providers to track urine flow, detect blockages, and prevent infections in real-time. These advancements are not only improving the effectiveness of nephrostomy procedures but also enhancing patient comfort and reducing the frequency of device-related complications. As technology continues to evolve, the nephrostomy device market is shifting toward more patient-centric and high-performance solutions.What Is Driving the Growth of the Nephrostomy Devices Market?
The growth in the nephrostomy devices market is driven by several factors, including the rising prevalence of kidney diseases, increasing adoption of minimally invasive procedures, and advancements in device technology. The global increase in chronic kidney disease (CKD), kidney stones, and urinary tract obstructions has created a growing demand for effective drainage solutions. Additionally, the aging population, which is more susceptible to urological disorders, is fueling market growth. The shift toward minimally invasive surgical techniques, particularly in urology, is also expanding the adoption of nephrostomy procedures, as these techniques reduce hospital stays, lower the risk of complications, and improve patient recovery times. Innovations in catheter materials, antimicrobial coatings, and remote monitoring capabilities are making nephrostomy devices more reliable and patient-friendly. Furthermore, improvements in healthcare infrastructure and increased investments in urology-focused medical research are expanding the availability of nephrostomy treatments, particularly in emerging economies. The expansion of reimbursement policies in various regions is also supporting market growth, making nephrostomy procedures more accessible to a larger patient population. As the demand for advanced urological interventions continues to rise, the nephrostomy devices market is poised for sustained expansion, offering improved treatment options for patients worldwide.Report Scope
The report analyzes the Nephrostomy Devices market, presented in terms of market value. The analysis covers the key segments and geographic regions outlined below.- Segments: Device Type (Guidewires, Drainage Tubes, Nephrostomy Catheters, Sheath Dilators, Other Product Types); End-Use (Hospitals End-Use, Emergency Clinics End-Use, Ambulatory Surgery Centers End-Use).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Guidewires segment, which is expected to reach US$635.3 Million by 2030 with a CAGR of a 4.9%. The Drainage Tubes segment is also set to grow at 7.9% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $387.9 Million in 2024, and China, forecasted to grow at an impressive 9.8% CAGR to reach $418.7 Million by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Nephrostomy Devices Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Nephrostomy Devices Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Nephrostomy Devices Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as Abbott Laboratories, B. Braun Melsungen AG, Boston Scientific Corporation, Chettawut Plastic Surgery Center, Conmed Corporation and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 36 companies featured in this Nephrostomy Devices market report include:
- AngioDynamics
- Argon Medical Devices, Inc.
- B. Braun Melsungen AG
- Becton, Dickinson and Company (BD)
- Boston Scientific Corporation
- Cardinal Health, Inc.
- Coloplast Group
- Cook Medical
- Envaste
- Halyard Health
- Meditech
- Medtronic plc
- Merit Medical Systems
- NeoMedical
- Olympus Corporation
- Rocamed
- Stryker Corporation
- Teleflex Incorporated
- Terumo Corporation
- UreSil LLC
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- AngioDynamics
- Argon Medical Devices, Inc.
- B. Braun Melsungen AG
- Becton, Dickinson and Company (BD)
- Boston Scientific Corporation
- Cardinal Health, Inc.
- Coloplast Group
- Cook Medical
- Envaste
- Halyard Health
- Meditech
- Medtronic plc
- Merit Medical Systems
- NeoMedical
- Olympus Corporation
- Rocamed
- Stryker Corporation
- Teleflex Incorporated
- Terumo Corporation
- UreSil LLC
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 279 |
| Published | February 2026 |
| Forecast Period | 2024 - 2030 |
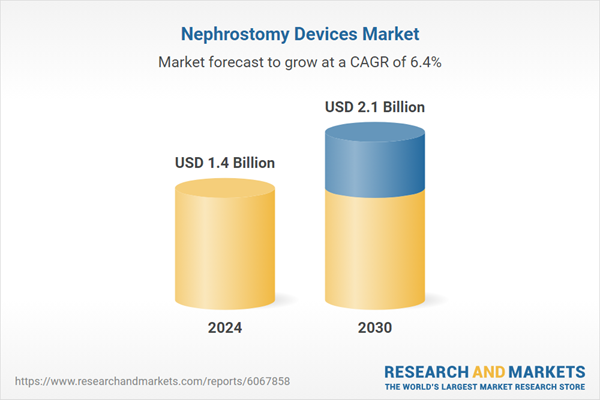
| Estimated Market Value ( USD | $ 1.4 Billion |
| Forecasted Market Value ( USD | $ 2.1 Billion |
| Compound Annual Growth Rate | 6.4% |
| Regions Covered | Global |