Global mRNA Therapeutics Market - Key Trends & Drivers Summarized
How Are mRNA Therapeutics Revolutionizing Drug Development and Disease Treatment?
The rise of mRNA therapeutics has fundamentally changed the landscape of drug development, offering a powerful platform for treating a wide range of diseases, from infectious diseases and cancer to genetic disorders and rare conditions. Unlike traditional biologics, which require complex production processes and cell culture systems, mRNA-based therapies leverage synthetic messenger RNA to instruct cells to produce therapeutic proteins directly within the body. This approach significantly accelerates drug development timelines and enhances precision in targeting disease pathways. The groundbreaking success of mRNA COVID-19 vaccines demonstrated the speed and flexibility of this technology, leading to a surge in research efforts aimed at expanding mRNA applications beyond vaccines. The ability to encode virtually any protein into an mRNA sequence has positioned this technology as a promising tool for personalized medicine, where treatments can be tailored to an individual’ s genetic profile. Additionally, advancements in lipid nanoparticle (LNP) delivery systems have improved the stability and targeted delivery of mRNA therapeutics, addressing previous challenges related to degradation and immunogenicity. As pharmaceutical companies and research institutions continue to invest in mRNA-based solutions, the potential for treating conditions such as autoimmune diseases, cardiovascular disorders, and neurodegenerative conditions is rapidly expanding, making mRNA therapeutics one of the most dynamic areas in modern biotechnology.What Technological Advancements Are Enhancing the Efficacy and Delivery of mRNA Drugs?
The rapid evolution of mRNA therapeutics is largely driven by advancements in formulation, delivery technologies, and sequence optimization. One of the most significant breakthroughs has been the development of chemically modified nucleotides, such as N1-methylpseudouridine, which enhance mRNA stability and reduce unwanted immune responses. Improved in vitro transcription (IVT) processes have increased mRNA yield and purity, reducing the presence of double-stranded RNA contaminants that can trigger inflammatory responses. The refinement of lipid nanoparticle (LNP) carriers has also played a crucial role in improving mRNA delivery, ensuring that the therapeutic payload reaches target tissues with high efficiency while minimizing off-target effects. Researchers are now exploring next-generation delivery systems, including polymer-based nanoparticles, exosome-derived vesicles, and peptide-based formulations, to further enhance tissue specificity and prolonged therapeutic effects. Additionally, the emergence of self-amplifying mRNA (saRNA) has opened new possibilities for lower-dose, longer-lasting treatments by enabling the production of therapeutic proteins at reduced mRNA concentrations. As artificial intelligence (AI) and machine learning are integrated into drug discovery, predictive modeling is being used to optimize mRNA sequences and delivery strategies, accelerating the development of novel mRNA-based therapies. These technological advancements are expected to expand the scope of mRNA therapeutics, unlocking new treatment paradigms across multiple disease areas.How Are Regulatory and Manufacturing Challenges Impacting the mRNA Therapeutics Market?
Despite the immense potential of mRNA therapeutics, the industry faces regulatory and manufacturing challenges that must be addressed to enable widespread adoption. The highly specialized nature of mRNA production requires stringent quality control measures, particularly in raw material sourcing, in vitro transcription, and purification processes. Regulatory agencies such as the FDA and EMA have introduced new guidelines for mRNA therapeutics, focusing on product stability, immunogenicity, and manufacturing scalability. The need for Good Manufacturing Practice (GMP)-compliant production facilities has increased demand for contract development and manufacturing organizations (CDMOs) that specialize in mRNA drug production. However, the global supply chain for key components such as nucleotides, capping reagents, and lipid carriers remains vulnerable to disruptions, posing risks to large-scale manufacturing. Additionally, regulatory frameworks are still evolving for personalized mRNA therapies, such as cancer vaccines, which require customized formulations for individual patients. Another key challenge is the cost of production, as mRNA-based drugs often require cold-chain storage and sophisticated logistics for global distribution. Addressing these challenges will require continued collaboration between biotech companies, regulatory agencies, and manufacturing partners to ensure that mRNA therapeutics can be developed and distributed efficiently and safely on a global scale.What Are the Key Growth Drivers Propelling the mRNA Therapeutics Market?
The growth in the mRNA therapeutics market is driven by several factors, including increasing investments in biotechnology research, expanding applications beyond vaccines, and technological advancements in mRNA delivery systems. The success of mRNA-based COVID-19 vaccines has significantly boosted funding for mRNA research, leading to an influx of clinical trials investigating mRNA therapies for cancer, metabolic diseases, and infectious diseases such as influenza, HIV, and Zika virus. The rise of personalized medicine has also accelerated interest in mRNA-based cancer vaccines, where patients receive customized treatments targeting specific tumor antigens. Additionally, the growing adoption of AI-driven drug discovery is streamlining mRNA sequence design, optimizing formulations, and reducing development timelines. The increasing demand for decentralized vaccine production and regional manufacturing hubs has spurred investments in mRNA production facilities, further supporting market expansion. The continued refinement of lipid nanoparticles and alternative delivery systems has enhanced the efficacy and safety of mRNA drugs, driving broader clinical adoption. Furthermore, government initiatives focused on pandemic preparedness and biopharmaceutical innovation are providing funding and regulatory support for mRNA therapeutics. As new indications emerge and manufacturing capabilities improve, the mRNA therapeutics market is poised for sustained growth, revolutionizing the treatment landscape for numerous diseases and positioning mRNA technology as a cornerstone of next-generation medicine.Report Scope
The report analyzes the mRNA Therapeutics market, presented in terms of market value. The analysis covers the key segments and geographic regions outlined below.- Segments: Type (Prophylactic Products, Therapeutic Products); Application (Infectious Diseases Application, Oncology Application, Rare Genetic Diseases Application, Respiratory Diseases Application, Other Applications); End-Use (Hospitals & Clinics End-Use, Research Organizations End-Use, Other End-Uses).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Prophylactic Products segment, which is expected to reach US$25.6 Billion by 2030 with a CAGR of a 18.3%. The Therapeutic Products segment is also set to grow at 14.2% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $3.6 Billion in 2024, and China, forecasted to grow at an impressive 22.8% CAGR to reach $7.7 Billion by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global mRNA Therapeutics Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global mRNA Therapeutics Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global mRNA Therapeutics Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as Aldevron, BioNTech SE, Bio-Synthesis Inc., BOC Sciences, Creative Biogene and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 48 companies featured in this mRNA Therapeutics market report include:
- Abogen Biosciences
- Alnylam Pharmaceuticals
- Anima Biotech
- Arcturus Therapeutics Holdings Inc.
- BioNTech SE
- Comanche Biopharma
- CureVac N.V.
- eTheRNA Immunotherapies NV
- Ethris GmbH
- EXACIS Biotherapeutics
- Gritstone bio, Inc.
- HC Bioscience
- Inflammatix, Inc.
- Ionis Pharmaceuticals
- Moderna, Inc.
- Nutcracker Therapeutics
- Orna Therapeutics
- ReCode Therapeutics
- Strand Therapeutics
- Translate Bio (acquired by Sanofi)
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Abogen Biosciences
- Alnylam Pharmaceuticals
- Anima Biotech
- Arcturus Therapeutics Holdings Inc.
- BioNTech SE
- Comanche Biopharma
- CureVac N.V.
- eTheRNA Immunotherapies NV
- Ethris GmbH
- EXACIS Biotherapeutics
- Gritstone bio, Inc.
- HC Bioscience
- Inflammatix, Inc.
- Ionis Pharmaceuticals
- Moderna, Inc.
- Nutcracker Therapeutics
- Orna Therapeutics
- ReCode Therapeutics
- Strand Therapeutics
- Translate Bio (acquired by Sanofi)
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 282 |
| Published | February 2026 |
| Forecast Period | 2024 - 2030 |
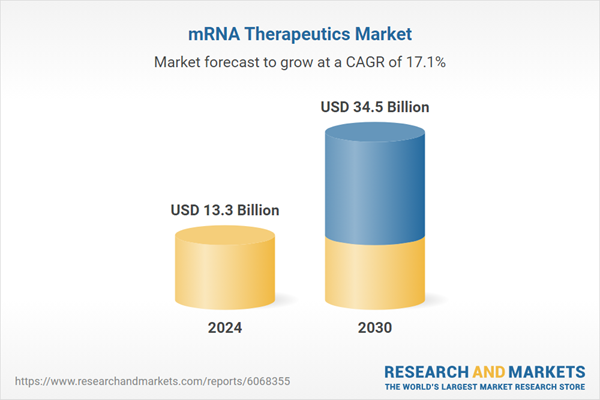
| Estimated Market Value ( USD | $ 13.3 Billion |
| Forecasted Market Value ( USD | $ 34.5 Billion |
| Compound Annual Growth Rate | 17.1% |
| Regions Covered | Global |