Global Small Volume Parenteral Market - Key Trends & Drivers Summarized
Why Is the Demand for Small Volume Parenteral Increasing? Understanding Their Role in Injectable Drug Delivery
Small volume parenteral (SVP) formulations play a crucial role in modern medicine, offering efficient and controlled drug delivery for a wide range of therapeutic applications. These injectable formulations, typically packaged in vials, ampoules, or prefilled syringes, are used for critical treatments such as vaccines, biologics, antibiotics, and pain management medications. The growing prevalence of chronic diseases, such as diabetes, cardiovascular conditions, and autoimmune disorders, has significantly increased the demand for SVPs, as many of these conditions require regular injectable treatments. Additionally, advancements in biologic drug development, including monoclonal antibodies and cell therapies, have further expanded the use of SVPs in personalized medicine. With healthcare providers emphasizing faster drug absorption and targeted therapy, the adoption of small volume parenteral solutions continues to rise in hospitals, clinics, and home healthcare settings.How Are Technological Innovations Transforming Small Volume Parenteral Manufacturing? Exploring Advancements in Aseptic Processing and Drug Stability
The manufacturing of small volume parenteral solutions has evolved with technological advancements in aseptic processing, packaging, and drug stability. Innovations in fill-finish technology, including closed-system aseptic filling, have improved sterility assurance while reducing contamination risks. Additionally, the use of advanced packaging materials, such as glass alternatives and polymer-based containers, has enhanced drug stability and shelf life. The rise of prefilled syringes and autoinjectors has further improved patient convenience, reducing the need for manual drug preparation and minimizing administration errors. AI-driven quality control systems and real-time monitoring technologies have also optimized production efficiency, ensuring regulatory compliance and product consistency. As pharmaceutical companies continue to invest in automation and smart manufacturing, the small volume parenteral market is witnessing improvements in cost-effectiveness, scalability, and safety.What Challenges Are Affecting the Small Volume Parenteral Market? Addressing Supply Chain Constraints, Regulatory Compliance, and Production Costs
Despite the growing demand for small volume parenteral formulations, the market faces challenges related to supply chain disruptions, regulatory hurdles, and high production costs. The pharmaceutical supply chain has been impacted by raw material shortages, particularly for glass vials, sterile injectables, and specialized excipients. Additionally, stringent regulatory requirements for parenteral manufacturing, including compliance with Good Manufacturing Practices (GMP) and sterility testing, require significant investment in quality control and facility upgrades. High production costs associated with aseptic processing, specialized storage conditions, and cold chain logistics further contribute to pricing pressures in the market. Addressing these challenges requires pharmaceutical companies to invest in diversified supply chains, continuous manufacturing technologies, and improved packaging solutions to enhance market resilience and efficiency.What's Driving the Growth of the Small Volume Parenteral Market? Identifying Key Expansion Factors and Industry Trends
The growth in the small volume parenteral market is driven by several factors, including the increasing prevalence of chronic and infectious diseases, rising demand for biologic drugs, and advancements in injectable drug delivery systems. The global expansion of immunization programs and the need for emergency medications have significantly boosted demand for SVPs, particularly in the vaccine and critical care segments. Additionally, the rise of self-administration devices, such as wearable injectors and autoinjectors, has improved patient adherence to injectable therapies, expanding the market potential for SVPs. The increasing adoption of biosimilars and personalized medicine has further fueled the need for precise and controlled parenteral formulations. As pharmaceutical innovations continue to enhance drug stability, sterility, and administration convenience, the small volume parenteral market is expected to experience sustained growth, playing a pivotal role in the future of injectable therapeutics.Report Scope
The report analyzes the Small Volume Parenteral market, presented in terms of market value (US$). The analysis covers the key segments and geographic regions outlined below:- Segments: Form (Liquid, Dry); Dose Type (Single-dose, Multiple-dose); Packaging Type (Ampoules, Vials, Pre-filled Syringes, Cartridges, Bottles, Others); Indication Type (Pain Management, Cancer Care, Malnourishment, Gastrointestinal disorder / Diarrhea, Diabetes, Others).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Some of the 42 companies featured in this Small Volume Parenteral market report include -
- AbbVie Contract Manufacturing
- Akums Drugs & Pharmaceuticals Ltd.
- Alcami Corporation
- Alembic Pharmaceuticals Ltd.
- B. Braun Melsungen AG
- Baxter International Inc.
- Becton, Dickinson and Company
- Bell-More Labs, Inc.
- Biocon Limited
- Bio-Concept Laboratories Inc.
- Catalent, Inc.
- Chemic Laboratories, Inc.
- DSM Pharmaceuticals Inc.
- Evonik Industries AG
- Fresenius Kabi AG
- Grifols Partnership
- Hikma Pharmaceuticals PLC
- ICU Medical, Inc.
- KP Pharmaceutical Technology Inc.
- Lupin Limited
- Patheon (Thermo Fisher Scientific)
- Pfizer Inc.
- Renaissance Lakewood LLC
- Sichuan Kelun Pharmaceutical Co., Ltd.
- Singota Solutions
- SL Pharma Labs Inc.
- Stevanato Group
- Torrent Pharmaceuticals Ltd.
- Wockhardt Ltd.
- Zydus Cadila
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Liquid Form segment, which is expected to reach US$192 Billion by 2030 with a CAGR of a 7.7%. The Dry Form segment is also set to grow at 4.6% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $51.3 Billion in 2024, and China, forecasted to grow at an impressive 10.7% CAGR to reach $58.1 Billion by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Small Volume Parenteral Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Small Volume Parenteral Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Small Volume Parenteral Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as Aenova Group, AGC Biologics, Alcami Corporation, Apeloa Pharmaceutical, Aurigene Pharmaceutical Services and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Select Competitors (Total 42 Featured):
- AbbVie Contract Manufacturing
- Akums Drugs & Pharmaceuticals Ltd.
- Alcami Corporation
- Alembic Pharmaceuticals Ltd.
- B. Braun Melsungen AG
- Baxter International Inc.
- Becton, Dickinson and Company
- Bell-More Labs, Inc.
- Biocon Limited
- Bio-Concept Laboratories Inc.
- Catalent, Inc.
- Chemic Laboratories, Inc.
- DSM Pharmaceuticals Inc.
- Evonik Industries AG
- Fresenius Kabi AG
- Grifols Partnership
- Hikma Pharmaceuticals PLC
- ICU Medical, Inc.
- KP Pharmaceutical Technology Inc.
- Lupin Limited
- Patheon (Thermo Fisher Scientific)
- Pfizer Inc.
- Renaissance Lakewood LLC
- Sichuan Kelun Pharmaceutical Co., Ltd.
- Singota Solutions
- SL Pharma Labs Inc.
- Stevanato Group
- Torrent Pharmaceuticals Ltd.
- Wockhardt Ltd.
- Zydus Cadila
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- AbbVie Contract Manufacturing
- Akums Drugs & Pharmaceuticals Ltd.
- Alcami Corporation
- Alembic Pharmaceuticals Ltd.
- B. Braun Melsungen AG
- Baxter International Inc.
- Becton, Dickinson and Company
- Bell-More Labs, Inc.
- Biocon Limited
- Bio-Concept Laboratories Inc.
- Catalent, Inc.
- Chemic Laboratories, Inc.
- DSM Pharmaceuticals Inc.
- Evonik Industries AG
- Fresenius Kabi AG
- Grifols Partnership
- Hikma Pharmaceuticals PLC
- ICU Medical, Inc.
- KP Pharmaceutical Technology Inc.
- Lupin Limited
- Patheon (Thermo Fisher Scientific)
- Pfizer Inc.
- Renaissance Lakewood LLC
- Sichuan Kelun Pharmaceutical Co., Ltd.
- Singota Solutions
- SL Pharma Labs Inc.
- Stevanato Group
- Torrent Pharmaceuticals Ltd.
- Wockhardt Ltd.
- Zydus Cadila
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 483 |
| Published | February 2026 |
| Forecast Period | 2024 - 2030 |
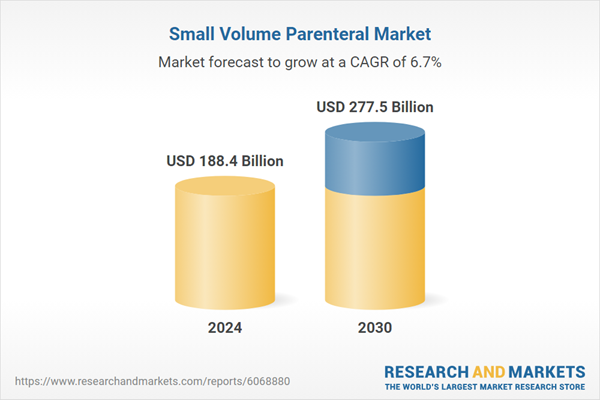
| Estimated Market Value ( USD | $ 188.4 Billion |
| Forecasted Market Value ( USD | $ 277.5 Billion |
| Compound Annual Growth Rate | 6.7% |
| Regions Covered | Global |