Global Hairy Cell Leukemia Market - Key Trends & Drivers Summarized
What Makes Hairy Cell Leukemia a Unique and Challenging Hematologic Malignancy?
Hairy Cell Leukemia (HCL) is a rare, indolent B-cell lymphoproliferative disorder that accounts for approximately 2% of all leukemias, predominantly affecting middle-aged to older adults, with a male-to-female ratio of nearly 4:1. It is characterized by the presence of abnormal B lymphocytes with cytoplasmic projections - resembling “ hair-like” structures - hence the name. Clinically, patients typically present with pancytopenia, splenomegaly, and recurrent infections due to immunosuppression. While the disease is slow-growing, its complexity lies in its subtle onset, diagnostic ambiguity, and potential for relapse. A hallmark of HCL is the presence of the BRAF V600E mutation, found in nearly 90% of classic cases, which has become a critical biomarker and therapeutic target. Diagnostically, HCL is confirmed using a combination of morphological assessment, flow cytometry, and immunophenotyping with markers like CD11c, CD25, CD103, and annexin A1. Bone marrow biopsies often reveal a “ dry tap” due to fibrosis, further complicating sample acquisition. Despite its slow progression, HCL significantly impacts quality of life through chronic fatigue, susceptibility to infections, and cytopenia-related complications. Its rarity makes randomized controlled trials challenging, often necessitating retrospective analyses and multi-center collaborations for meaningful data generation. The disease’ s slow course, overlapping symptoms with other hematological malignancies, and variable response to treatment necessitate a nuanced clinical approach, combining targeted therapies with long-term surveillance. Research and clinical interest in HCL have grown, largely due to breakthroughs in genomics and targeted drug development, positioning it as a distinct and strategically significant area within hematologic oncology.How Have Treatment Paradigms for Hairy Cell Leukemia Evolved Over Time?
The therapeutic landscape for Hairy Cell Leukemia has witnessed significant shifts over the past four decades, moving from palliative splenectomy toward precision medicine. The introduction of purine analogs such as cladribine and pentostatin in the late 1980s revolutionized HCL management, achieving durable complete responses in a large proportion of patients and transforming a once-lethal disease into a chronic but manageable condition. Cladribine remains a frontline agent due to its high response rate and relatively well-tolerated toxicity profile. However, relapsed or refractory HCL cases have posed a persistent challenge, especially those unresponsive to purine analogs. This has led to the development and regulatory approval of newer therapies such as moxetumomab pasudotox - a CD22-directed cytotoxin - and BRAF inhibitors like vemurafenib, either as monotherapy or in combination with rituximab. These newer agents specifically target the underlying molecular drivers of HCL and have shown efficacy in patients with multiple relapses or treatment resistance. Immunotherapy is gaining traction in HCL treatment strategies, particularly monoclonal antibodies targeting CD20 (rituximab), CD22, and bispecific T-cell engagers under investigation. Hematopoietic stem cell transplantation, although rarely used, is considered in highly refractory cases. The growing interest in combination regimens seeks to enhance remission durability while minimizing cumulative toxicity, especially in elderly or comorbid populations. Furthermore, patient management now incorporates regular monitoring of minimal residual disease (MRD) using highly sensitive molecular assays, enabling earlier interventions and personalized treatment planning. These evolving therapeutic strategies underscore the shift from cytotoxic, broadly acting drugs toward biomarker-driven precision medicine in HCL care.What Diagnostic Innovations and Research Trends Are Steering the Market Forward?
Technological advancements in diagnostic methodologies have substantially improved the accuracy, speed, and sensitivity of Hairy Cell Leukemia detection, aiding both early diagnosis and post-treatment surveillance. The integration of high-resolution flow cytometry and next-generation sequencing (NGS) into routine diagnostic workflows allows for detailed immunophenotypic profiling and mutation analysis, particularly the BRAF V600E mutation, which serves both diagnostic and therapeutic functions. Immunohistochemistry and PCR-based assays further aid in differentiating classic HCL from its variants, such as HCL-variant (HCL-v) and splenic marginal zone lymphoma, which are morphologically and clinically distinct yet often confused. Emerging single-cell RNA sequencing and digital droplet PCR technologies are being explored for their potential to detect MRD at unprecedented sensitivity, facilitating more precise disease monitoring and timely therapeutic adjustments. AI-based diagnostic platforms are also making inroads, offering predictive analytics based on large datasets encompassing genetic profiles, clinical features, and treatment responses. On the research front, there is increasing focus on understanding the microenvironmental interactions within the bone marrow niche that support HCL cell survival. Investigational therapies targeting MEK, ERK, and other elements downstream of the BRAF pathway are currently in early-phase trials, aimed at overcoming resistance and enhancing therapeutic durability. Biobanking initiatives and international HCL registries are expanding, enabling better longitudinal data collection and real-world evidence generation. As clinical guidelines become more sophisticated, there is a growing emphasis on stratifying patients not just by disease subtype but also by genetic, molecular, and treatment response profiles, paving the way for a more personalized and outcomes-driven approach in HCL management.The Growth In The Global Hairy Cell Leukemia Market Is Driven By Several Factors…
The growth in the global Hairy Cell Leukemia market is driven by several factors tied to diagnostic innovation, precision therapeutics, increasing disease surveillance, and evolving patient care paradigms. A primary growth driver is the expanded accessibility of molecular diagnostics, particularly BRAF V600E mutation testing, which has enabled faster and more accurate disease classification, improving treatment outcomes. Rising awareness among clinicians and patients, combined with greater availability of hematologic consultation in emerging markets, has led to earlier diagnosis and more proactive treatment initiation. The continued approval and uptake of targeted therapies such as vemurafenib and moxetumomab pasudotox are expanding the therapeutic arsenal, particularly for relapsed and refractory cases. Additionally, the emergence of combination regimens involving purine analogs and monoclonal antibodies is extending remission duration and reducing relapse rates, creating long-term value in care delivery. Increasing adoption of MRD monitoring is also reshaping clinical decision-making, driving demand for advanced molecular testing platforms. From a healthcare infrastructure perspective, the growing penetration of specialized oncology centers and increased hematology training in developing countries are improving patient access to state-of-the-art care. Regulatory incentives such as orphan drug status and fast-track designations are encouraging pharmaceutical innovation and reducing time-to-market for novel agents. Moreover, the integration of HCL-specific clinical trials within broader hematologic oncology networks is accelerating data accumulation and best-practice dissemination. Lastly, the broader trend toward individualized cancer care, supported by electronic medical records and real-world evidence, is enhancing treatment adherence and outcome optimization. These interlinked developments - rooted in technology, access, therapeutics, and health system modernization - are fueling the steady expansion of the global Hairy Cell Leukemia market.Report Scope
The report analyzes the Hairy Cell Leukemia market, presented in terms of market value. The analysis covers the key segments and geographic regions outlined below.- Segments: Therapy (Chemotherapy, Targeted Therapy); Gender (Male Gender, Female Gender).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Chemotherapy segment, which is expected to reach US$148.8 Billion by 2030 with a CAGR of a 3.7%. The Targeted Therapy segment is also set to grow at 3.6% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $51.5 Billion in 2024, and China, forecasted to grow at an impressive 6.8% CAGR to reach $47.1 Billion by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Hairy Cell Leukemia Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Hairy Cell Leukemia Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Hairy Cell Leukemia Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as Ace High Co, Amorepacific Corporation, Avon Products, Inc., CHI Haircare (Farouk Systems), Coty Inc. and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 42 companies featured in this Hairy Cell Leukemia market report include:
- Amega Biotech
- Amgen Inc.
- Astellas Pharma Inc.
- AstraZeneca PLC
- Beam Therapeutics
- bluebird bio
- Dr. Reddy's Laboratories
- Emcure Pharmaceuticals
- F. Hoffmann-La Roche Ltd
- Genentech (a member of the Roche Group)
- Gilead Sciences, Inc.
- Incyte Corporation
- Innate Pharma
- Johnson & Johnson
- Merck KGaA
- Mission Bio
- Novartis AG
- Pfizer Inc.
- Roivant Sciences
- Takeda Pharmaceutical Company Limited
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Amega Biotech
- Amgen Inc.
- Astellas Pharma Inc.
- AstraZeneca PLC
- Beam Therapeutics
- bluebird bio
- Dr. Reddy's Laboratories
- Emcure Pharmaceuticals
- F. Hoffmann-La Roche Ltd
- Genentech (a member of the Roche Group)
- Gilead Sciences, Inc.
- Incyte Corporation
- Innate Pharma
- Johnson & Johnson
- Merck KGaA
- Mission Bio
- Novartis AG
- Pfizer Inc.
- Roivant Sciences
- Takeda Pharmaceutical Company Limited
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 273 |
| Published | February 2026 |
| Forecast Period | 2024 - 2030 |
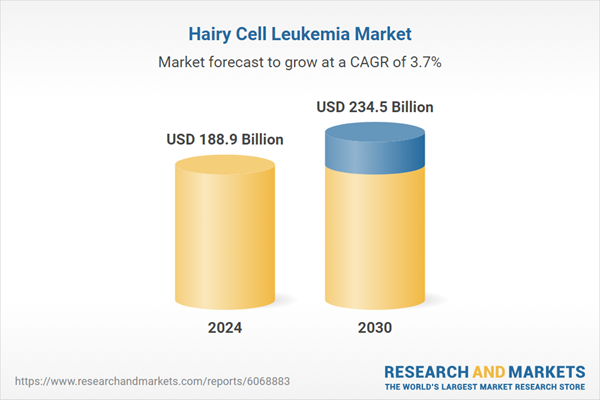
| Estimated Market Value ( USD | $ 188.9 Billion |
| Forecasted Market Value ( USD | $ 234.5 Billion |
| Compound Annual Growth Rate | 3.7% |
| Regions Covered | Global |