Global Pediatric Clinical Trials Market - Key Trends & Drivers Summarized
Why Is Pediatric-Specific Research No Longer Optional in the Drug Development Landscape?
Pediatric clinical trials have transitioned from a peripheral element of drug development to a regulatory and ethical imperative, reflecting a growing consensus that children require distinct scientific evaluation rather than mere extrapolation from adult studies. Historically, a significant portion of drugs prescribed to pediatric patients were off-label, lacking pediatric-specific safety and efficacy data, which introduced considerable risk and uncertainty into treatment protocols. Today, international regulatory frameworks such as the U.S. FDA's Pediatric Research Equity Act (PREA), the EU Pediatric Regulation, and similar initiatives across Asia-Pacific are mandating age-appropriate studies as part of new drug applications. This shift has led to a steady increase in pediatric clinical trials across therapeutic areas including oncology, rare genetic disorders, neurology, endocrinology, infectious diseases, and respiratory care. These trials must address complex considerations - age-appropriate dosing, developmental pharmacokinetics, formulation design, ethical consent, and long-term outcome monitoring - that demand specialized protocols and infrastructure. As such, pediatric trial execution has become a niche specialization requiring tailored investigator training, pediatric-focused clinical research organizations (CROs), and the involvement of pediatric patient advocacy groups. Major pharmaceutical companies are establishing dedicated pediatric centers of excellence to integrate child-centric research earlier in the drug development lifecycle. Moreover, with a global rise in early disease detection through newborn screening and genetic testing, clinical trial designs are evolving to accommodate both acute and preventive investigational therapies in pediatric populations. The increasing institutionalization of pediatric research is not just a regulatory requirement - it is a transformative shift toward age-inclusive evidence-based medicine.How Are Trial Designs and Regulatory Innovations Reshaping the Pediatric Research Model?
Advancements in clinical trial methodologies and regulatory strategies are playing a transformative role in making pediatric research more agile, precise, and ethically sound. Traditional clinical trial models are often ill-suited for pediatric populations due to recruitment challenges, small patient pools, and the ethical sensitivity of involving children in experimental treatments. In response, regulators and sponsors are embracing adaptive trial designs, Bayesian statistics, and real-world evidence (RWE) models to extract meaningful data from smaller, stratified cohorts. Pediatric trials are increasingly incorporating decentralized and hybrid frameworks, leveraging telehealth, wearable sensors, and remote data capture to reduce the burden on participants and their families. These models are proving particularly valuable in rare disease research, where patient populations are geographically dispersed and enrollment must be maximized across borders. Simultaneously, age de-escalation strategies, where trials begin with adolescents and gradually include younger children and infants, are helping mitigate safety risks and refine dosing protocols. On the regulatory front, agencies are offering fast-track designations, pediatric investigation plans (PIPs), and incentives like extended market exclusivity for companies that proactively pursue pediatric indications. Ethics committees are becoming more streamlined and globally harmonized, facilitating faster multi-site trial approvals. Additionally, digital platforms for e-consent and child-friendly trial education materials are helping overcome parental hesitancy and enhance informed participation. These innovations are not merely administrative conveniences - they are enabling more efficient, scalable, and compliant pediatric trials that maintain scientific rigor while respecting the unique needs of children and their caregivers.Why Are Sponsors and CROs Investing More Aggressively in Pediatric Trial Infrastructure?
The operational landscape of pediatric clinical trials is being reshaped by growing sponsor interest and a surge in pediatric-focused infrastructure development. Clinical research organizations (CROs) are expanding their pediatric portfolios, recruiting child-specific principal investigators, and forming dedicated pediatric project management teams to serve the complex logistical, regulatory, and therapeutic demands of youth-focused trials. Leading sponsors are investing in pediatric research hubs and trial networks within children's hospitals and academic medical centers, recognizing that high-quality data and enrollment efficiency depend heavily on access to specialized clinical sites. There is also a strong movement toward globalizing pediatric trials, particularly across emerging markets such as India, Brazil, and Southeast Asia, where large pediatric populations and expanding healthcare infrastructure offer untapped potential for recruitment. These expansions are supported by governments and NGOs that are facilitating trial site development and capacity-building initiatives. Moreover, the rise in rare pediatric diseases has catalyzed collaboration between pharmaceutical companies, non-profit foundations, and patient advocacy groups to co-develop trial protocols, natural history databases, and registries. Integration of genomic and biomarker-driven eligibility criteria is also refining patient selection, particularly in oncology and neurology, enabling more targeted and meaningful outcomes. Technological infrastructure, such as electronic data capture (EDC), remote monitoring platforms, and digital patient engagement tools, is enhancing real-time oversight and compliance. Collectively, these efforts are streamlining trial operations, reducing cycle times, and increasing the scalability of pediatric trials. This infrastructure transformation reflects a long-term strategic shift, positioning pediatric trials as a core pillar of future clinical development pipelines rather than a regulatory afterthought.The Growth in the Pediatric Clinical Trials Market Is Driven by Several Factors…
The growth in the pediatric clinical trials market is driven by several factors anchored in regulatory mandates, therapeutic innovation, and evolving trial execution models. One of the foremost drivers is the increasing regulatory pressure to include pediatric populations in new drug and biologic development programs, prompting sponsors to initiate parallel pediatric plans early in the product lifecycle. Simultaneously, the expansion of precision medicine and gene therapy pipelines targeting pediatric-onset diseases - particularly in oncology, neurology, and rare genetic disorders - is necessitating age-stratified trials with pediatric-specific endpoints. On the end-use front, children's hospitals, academic pediatric centers, and global research networks are investing in trial-ready infrastructure, including pediatric biobanks, genomic screening tools, and centralized ethics review mechanisms, accelerating trial setup and execution. Advances in pediatric formulation science are another key growth factor, enabling age-appropriate delivery systems such as mini-tablets, dissolvable films, and taste-masked liquids that support trial adherence and safety. Additionally, digital transformation is playing a vital role - remote patient monitoring, decentralized trial platforms, and AI-based patient matching are improving recruitment, retention, and real-time data analysis. From a consumer standpoint, heightened parental awareness, advocacy group activism, and growing public trust in pediatric research are enhancing enrollment rates and trial transparency. There is also increasing alignment between sponsors and healthcare providers to integrate research with routine care, allowing real-world evidence generation within standard pediatric practice. As biopharma companies seek to differentiate their pipelines and fulfill unmet needs in pediatric populations, the clinical trials segment is experiencing an infusion of funding, innovation, and strategic prioritization. These converging dynamics are not only accelerating the pace of pediatric drug development but are also laying the foundation for a more inclusive, data-driven, and child-centric global clinical research ecosystem.Report Scope
The report analyzes the Pediatric Clinical Trials market, presented in terms of market value (US$). The analysis covers the key segments and geographic regions outlined below:- Segments: Phase (Phase I, Phase II, Phase III, Phase IV); Study Design (Treatment Studies, Observational Studies); Indication (Infectious Diseases, Oncology, Autoimmune / inflammation, Respiratory Disorders, Mental Health Disorders, Others).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Phase I Clinical Trials segment, which is expected to reach US$9.9 Billion by 2030 with a CAGR of a 2.8%. The Phase II Clinical Trials segment is also set to grow at 4.7% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $5.3 Billion in 2024, and China, forecasted to grow at an impressive 6.8% CAGR to reach $4.8 Billion by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Pediatric Clinical Trials Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Pediatric Clinical Trials Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Pediatric Clinical Trials Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as AbbVie Inc., Bristol-Myers Squibb Company, Charles River Laboratories International Inc., Covance Inc. (now part of Labcorp), Eli Lilly and Company and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 47 companies featured in this Pediatric Clinical Trials market report include:
- AbbVie Inc.
- Bristol-Myers Squibb Company
- Charles River Laboratories International Inc.
- Covance Inc. (now part of Labcorp)
- Eli Lilly and Company
- GlaxoSmithKline plc (GSK)
- ICON plc
- IQVIA Holdings Inc.
- Johnson & Johnson
- Medpace, Inc.
- Novartis AG
- Parexel International Corporation
- Pfizer Inc.
- PPD Inc. (Pharmaceutical Product Development)
- PRA Health Sciences (now part of ICON plc)
- Premier Research
- Sanofi S.A.
- Syneos Health Inc.
- Takeda Pharmaceutical Company Limited
- The Emmes Company, LLC
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- AbbVie Inc.
- Bristol-Myers Squibb Company
- Charles River Laboratories International Inc.
- Covance Inc. (now part of Labcorp)
- Eli Lilly and Company
- GlaxoSmithKline plc (GSK)
- ICON plc
- IQVIA Holdings Inc.
- Johnson & Johnson
- Medpace, Inc.
- Novartis AG
- Parexel International Corporation
- Pfizer Inc.
- PPD Inc. (Pharmaceutical Product Development)
- PRA Health Sciences (now part of ICON plc)
- Premier Research
- Sanofi S.A.
- Syneos Health Inc.
- Takeda Pharmaceutical Company Limited
- The Emmes Company, LLC
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 389 |
| Published | January 2026 |
| Forecast Period | 2024 - 2030 |
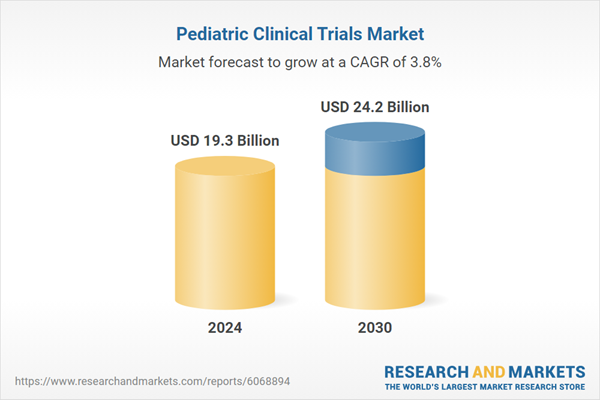
| Estimated Market Value ( USD | $ 19.3 Billion |
| Forecasted Market Value ( USD | $ 24.2 Billion |
| Compound Annual Growth Rate | 3.8% |
| Regions Covered | Global |