Global Omics-Based Clinical Trials Market - Key Trends & Drivers Summarized
Why Are Omics-Based Clinical Trials Redefining Drug Development and Precision Medicine?
Omics-based clinical trials are transforming the landscape of drug development by offering unprecedented insights into the molecular underpinnings of disease and therapeutic response. By integrating genomic, transcriptomic, proteomic, metabolomic, and epigenomic data into trial design and patient stratification, these trials enable more targeted, predictive, and efficient approaches to evaluating drug efficacy and safety. Unlike conventional trials, which rely heavily on generalized population-based models, omics-informed trials identify specific biomarkers or molecular signatures that correlate with treatment response, allowing for the selection of subpopulations most likely to benefit from a therapy. This reduces trial failure rates, accelerates timelines, and enhances clinical outcomes. In oncology, for example, next-generation sequencing (NGS) is routinely used to match patients to targeted therapies or immunotherapies based on their tumor’ s genetic mutations. Similar approaches are gaining traction in neurology, immunology, and rare disease studies. As personalized medicine becomes central to healthcare, omics-based clinical trials are becoming indispensable tools for pharmaceutical and biotech companies seeking to develop therapies that are not only effective but also tailored to individual patient profiles. Their ability to integrate molecular diagnostics into therapeutic evaluation is reshaping the very framework of modern clinical research.How Is Technology Enabling More Efficient, Multi-Dimensional Trial Design?
Rapid advancements in omics technologies and computational tools are significantly enhancing the design and execution of omics-based clinical trials. High-throughput sequencing platforms, mass spectrometry, and single-cell analysis are enabling deeper and more precise characterization of biological samples across multiple omics layers. At the same time, big data analytics, artificial intelligence (AI), and machine learning (ML) are revolutionizing the way omics data is interpreted and used to drive decision-making during clinical trials. These tools facilitate the identification of predictive biomarkers, the clustering of patient subgroups based on molecular phenotypes, and the optimization of dosage and treatment regimens. Digital health platforms are now integrating real-time omics data with clinical endpoints, wearable sensor data, and electronic health records (EHRs), enabling continuous and adaptive trial models. Cloud-based bioinformatics infrastructure allows for secure, scalable, and collaborative data processing, making global, multi-center omics trials more feasible than ever before. Furthermore, advances in sample preservation and logistics are streamlining biospecimen handling, ensuring data quality from source to analysis. These technological innovations are not only driving the feasibility and accuracy of omics-based trials but are also empowering a more agile, adaptive, and cost-effective clinical research environment that meets the demands of modern therapeutic innovation.Is Regulatory Support and Industry Collaboration Accelerating Omics-Based Trial Adoption?
Growing support from regulatory agencies and strategic collaborations among industry players are significantly accelerating the adoption of omics-based clinical trials. Regulatory bodies like the FDA, EMA, and PMDA are increasingly open to biomarker-driven trial designs, particularly in oncology and rare disease research, where traditional trial structures often fall short. The FDA’ s support for Real-Time Oncology Review (RTOR) and breakthrough therapy designations has created a more favorable regulatory climate for omics-based approaches. Moreover, regulatory frameworks are evolving to accommodate adaptive trial designs, companion diagnostics, and the co-development of therapeutic- diagnostic pairs, enabling faster approval pathways for personalized treatments. Strategic partnerships between pharmaceutical companies, diagnostics firms, academic institutions, and contract research organizations (CROs) are playing a pivotal role in creating shared databases, validating biomarkers, and building integrated omics platforms that support large-scale clinical studies. Industry-wide initiatives such as the Cancer Moonshot and the All of Us Research Program are providing rich datasets and infrastructure for omics-driven research. Furthermore, patient advocacy groups are increasingly involved in trial design and recruitment, particularly for rare and genetic diseases, ensuring that trials are patient-centered and inclusive. As regulatory frameworks become more responsive and ecosystems more collaborative, omics-based clinical trials are gaining momentum as the gold standard for personalized drug development and therapeutic validation.What’ s Driving the Global Expansion of the Omics-Based Clinical Trials Market?
The growth in the omics-based clinical trials market is driven by several interrelated factors rooted in technological innovation, unmet clinical needs, evolving therapeutic models, and increased investment in precision medicine. One of the primary drivers is the escalating demand for targeted therapies, especially in oncology, immunotherapy, and rare diseases, where traditional one-size-fits-all approaches have limited efficacy. The explosion of genomic and multi-omics data is enabling researchers to design trials that are more selective, hypothesis-driven, and efficient, improving the probability of clinical success. Additionally, pharmaceutical and biotech companies are increasingly investing in omics-based platforms to de-risk drug pipelines, optimize patient recruitment, and gain competitive advantage through biomarker-driven product differentiation. The increasing affordability and accessibility of NGS and bioinformatics tools are democratizing omics trial capabilities across mid-sized companies and academic research institutions. Meanwhile, global initiatives promoting population genomics and personalized medicine - particularly in the U.S., U.K., China, and the Middle East - are creating a robust infrastructure and funding base for large-scale omics-enabled trials. Regulatory encouragement for adaptive trial designs and real-world evidence integration is also shortening approval timelines and boosting industry confidence. Furthermore, rising public awareness around personalized health, genetic testing, and disease prevention is creating a more engaged and informed patient population, facilitating recruitment and retention in biomarker-based studies. Together, these dynamics are powering the global expansion of the omics-based clinical trials market, cementing its role as a transformative force in next-generation drug development and individualized healthcare.Report Scope
The report analyzes the Omics-based Clinical Trials market, presented in terms of market value. The analysis covers the key segments and geographic regions outlined below.- Segments: Phase (Phase I, Phase II, Phase III, Phase IV); Study (Interventional Studies, Observational Studies, Expanded Access Studies); Indication (Oncology Indication, Cardiovascular Indication, CNS Conditions Indication, Pain Management Indication, Diabetes Indication, Autoimmune / Inflammation Indication, Obesity Indication, Other Indications).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; and Rest of Europe); Asia-Pacific; Rest of World.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Phase I Clinical Trials segment, which is expected to reach US$23.3 Billion by 2030 with a CAGR of a 8.6%. The Phase II Clinical Trials segment is also set to grow at 5.1% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $9.3 Billion in 2024, and China, forecasted to grow at an impressive 7% CAGR to reach $8.6 Billion by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Omics-based Clinical Trials Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Omics-based Clinical Trials Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Omics-based Clinical Trials Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as Agilent Technologies, Inc., BD (Becton, Dickinson and Company), Beijing Genomics Institute (BGI), Biomarker Technologies, Bruker Corporation and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 32 companies featured in this Omics-based Clinical Trials market report include:
- Abbott Laboratories
- Agilent Technologies, Inc.
- Bristol-Myers Squibb Company
- Charles River Laboratories
- Covance Inc.
- Eli Lilly and Company
- Fulgent Genetics
- ICON plc
- Illumina, Inc.
- Laboratory Corporation of America
- Novo Nordisk A/S
- Parexel International Corporation
- PerkinElmer, Inc.
- Pfizer Inc.
- Pharmaceutical Product Development (PPD)
- Q2 Solutions
- Rebus Biosystems, Inc.
- SGS Société Générale de Surveillance SA
- Siemens Healthineers
- Thermo Fisher Scientific Inc.
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Abbott Laboratories
- Agilent Technologies, Inc.
- Bristol-Myers Squibb Company
- Charles River Laboratories
- Covance Inc.
- Eli Lilly and Company
- Fulgent Genetics
- ICON plc
- Illumina, Inc.
- Laboratory Corporation of America
- Novo Nordisk A/S
- Parexel International Corporation
- PerkinElmer, Inc.
- Pfizer Inc.
- Pharmaceutical Product Development (PPD)
- Q2 Solutions
- Rebus Biosystems, Inc.
- SGS Société Générale de Surveillance SA
- Siemens Healthineers
- Thermo Fisher Scientific Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 176 |
| Published | January 2026 |
| Forecast Period | 2024 - 2030 |
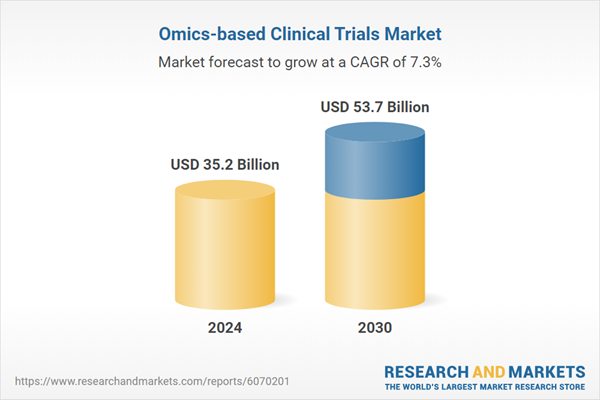
| Estimated Market Value ( USD | $ 35.2 Billion |
| Forecasted Market Value ( USD | $ 53.7 Billion |
| Compound Annual Growth Rate | 7.3% |
| Regions Covered | Global |