Global Endotherapy Devices Market - Key Trends & Drivers Summarized
What Is Driving the Strategic Importance of Endotherapy Devices in Modern Healthcare?
The endotherapy devices market has emerged as one of the most strategically important components of the endoscopy landscape, propelled by the rapid transformation of endoscopy from a diagnostic tool to a therapeutic platform. These devices, including snares, stents, hemostatic clips, dilation balloons, retrieval baskets, electrosurgical tools, and ablation systems, are instrumental in enabling minimally invasive treatment of gastrointestinal, pulmonary, urological, and biliary disorders. Their application spans a wide clinical spectrum - from polypectomy and gastrointestinal bleeding management to endoscopic stricturoplasty and tumor resection - allowing patients to avoid more invasive surgical alternatives. As minimally invasive interventions gain ground globally, endotherapy devices are playing an increasingly central role in expanding procedural capabilities and enabling same-day therapeutic care across high-volume centers and ambulatory surgical facilities.The rising burden of chronic GI conditions, such as colorectal cancer, inflammatory bowel disease (IBD), and GERD, alongside the growing prevalence of obesity-related disorders, has amplified the need for advanced endoscopic therapeutic solutions. Endotherapy is now considered a front-line option in managing early-stage cancers, functional GI disorders, and biliary obstructions, especially in patients unsuitable for open surgery. These devices are being adopted across diverse care settings, from tertiary hospitals and academic centers to standalone GI clinics. As health systems push for shorter hospital stays, reduced complications, and faster recovery times, the demand for versatile, high-efficacy endotherapy solutions is accelerating globally - with market expansion reinforced by reimbursement coverage improvements, increased procedural training, and better integration of these devices into value-based care models.
How Are Technological Advancements Enhancing Therapeutic Precision and Outcomes?
The evolution of endotherapy devices is tightly interwoven with advancements in material science, design innovation, and energy delivery technologies. Manufacturers are introducing devices with improved maneuverability, reduced profile diameters, and enhanced precision to navigate complex anatomies and enable tissue-specific intervention. Electrosurgical knives and ablation catheters are now engineered with insulation controls, smart energy feedback systems, and multi-functional tips to allow coagulation, dissection, and resection in a single tool. These advancements are not only reducing procedure time but also minimizing risk and improving reproducibility across varying skill levels.Smart therapeutic systems are also gaining traction. Examples include self-expanding stents with anti-migration features, drug-eluting variants for oncology applications, and balloon catheters with integrated sensors to regulate pressure and temperature. Advanced retrieval devices, like rotatable baskets and magnetic probes, are facilitating safer foreign body extraction and stent removal. The convergence of endotherapy with digital endoscopy and AI-assisted navigation systems is enabling more precise localization and deployment of devices. Moreover, the integration of disposable and semi-disposable therapeutic tools is improving infection control and procedural efficiency - a factor of growing importance in ambulatory and outpatient care settings. As new endoscopic techniques such as ESD, POEM, and NOTES mature, the sophistication and specificity of the endotherapy tools designed to support them continue to advance, reshaping the boundaries of what can be achieved through flexible endoscopy.
Which Clinical Areas and Applications Are Fueling Market Expansion?
The widespread applicability of endotherapy devices across multiple disciplines is a core engine of market growth. In gastroenterology, where these tools are most heavily used, high-volume procedures such as polypectomies, gastrointestinal bleeding control, and stent placements for malignant or benign strictures are driving daily utilization. With the increasing adoption of colorectal cancer screening programs worldwide, demand for snare-based polypectomy and endoscopic mucosal resection (EMR) kits has surged. Similarly, the growth in bariatric endoscopy has introduced a new set of requirements for tissue approximation tools, suturing systems, and closure devices. Electrosurgical solutions are also gaining favor in managing conditions such as Barrett’ s esophagus through radiofrequency ablation or cryotherapy-assisted procedures.Beyond GI, urology and pulmonology are emerging as high-growth areas for endotherapy device adoption. Urologists are using balloon dilation and self-expanding metallic stents (SEMS) for managing urethral strictures and urinary tract obstructions, while pulmonologists are increasingly using stents, forceps, and retrieval devices in interventional bronchoscopy. Biliary endoscopy, particularly in ERCP procedures, is experiencing a surge in demand for retrieval baskets, dilation balloons, and wire-guided cannulation accessories. Meanwhile, the shift toward day-case procedures is reinforcing demand for single-use, easy-to-deploy tools that can support fast turnover without compromising safety. The expansion of procedural scope in both established and emerging specialties is enabling device manufacturers to capture diversified revenue streams and position their products across multiple clinical endpoints.
What Are the Core Drivers Accelerating Global Market Growth Today?
The growth in the endotherapy devices market is driven by several factors centered on technological evolution, expanding end-use applications, and procedural migration toward minimally invasive therapeutic endoscopy. Technological innovations such as multi-functional electrosurgical tools, drug-eluting and self-expanding stents, advanced hemostatic agents, and sensor-embedded dilation balloons are enabling more complex interventions to be performed endoscopically. These innovations are expanding the procedural envelope and supporting safer, more efficient treatment across a broader patient base.End-use diversification is another major driver, as demand increases across gastroenterology, pulmonology, urology, and bariatrics. Each specialty presents unique anatomical and procedural challenges, necessitating the development of customized toolsets tailored to specific disease conditions and surgical objectives. In parallel, the rise of outpatient endoscopy centers, ambulatory surgical facilities, and specialty GI clinics is creating new demand for portable, sterile, and single-use therapeutic tools. Procedural growth is also being accelerated by healthcare infrastructure investments in Asia-Pacific, the Middle East, and Latin America, where public and private sector partnerships are equipping hospitals with advanced endoscopy capabilities. In mature markets, reimbursement reforms and value-based care incentives are aligning with trends in early diagnosis and intervention, reinforcing the commercial case for endotherapy device adoption. These factors collectively position the market for sustained global growth - supported by continued innovation, clinical expansion, and cross-specialty integration.
Report Scope
The report analyzes the Endotherapy Devices market, presented in terms of market value. The analysis covers the key segments and geographic regions outlined below.- Segments: Product (Gastrointestinal devices & accessories, Endoscopic retrograde cholangio pancreatography devices & accessories, Other products); End-Use (Hospitals, Ambulatory Surgery Centers, Others).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Gastrointestinal Devices & Accessories segment, which is expected to reach US$3.8 Billion by 2030 with a CAGR of a 4.6%. The Endoscopic Retrograde Cholangio Pancreatography Devices & Accessories segment is also set to grow at 7.8% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $1.4 Billion in 2024, and China, forecasted to grow at an impressive 9% CAGR to reach $1.4 Billion by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Endotherapy Devices Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Endotherapy Devices Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Endotherapy Devices Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 32 companies featured in this Endotherapy Devices market report include:
- Boston Scientific Corporation
- ConMed Corporation
- Cook Medical, LLC
- FUJIFILM Holdings Corporation
- Hoya Corporation (Pentax Medical)
- Intuitive Surgical
- M.I.Tech Co., Ltd.
- Medtronic Plc
- Micro-Tech (Nanjing) Co., Ltd
- Olympus Medical
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Boston Scientific Corporation
- ConMed Corporation
- Cook Medical, LLC
- FUJIFILM Holdings Corporation
- Hoya Corporation (Pentax Medical)
- Intuitive Surgical
- M.I.Tech Co., Ltd.
- Medtronic Plc
- Micro-Tech (Nanjing) Co., Ltd
- Olympus Medical
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 269 |
| Published | February 2026 |
| Forecast Period | 2024 - 2030 |
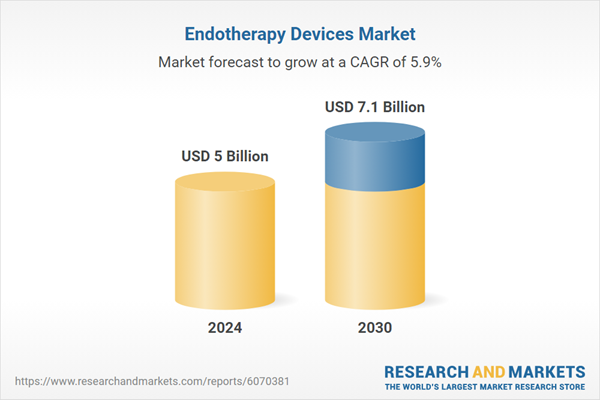
| Estimated Market Value ( USD | $ 5 Billion |
| Forecasted Market Value ( USD | $ 7.1 Billion |
| Compound Annual Growth Rate | 5.9% |
| Regions Covered | Global |