Global Small Molecule CDMO Market - Key Trends & Drivers Summarized
Why Are Small Molecule CDMOs Gaining Traction? Examining Their Role in Pharmaceutical Drug Development and Manufacturing
The increasing complexity of drug development and the rising demand for specialized contract development and manufacturing organizations (CDMOs) have positioned small molecule CDMOs as vital players in the pharmaceutical industry. Small molecules, which form the backbone of traditional drug formulations, continue to dominate the pharmaceutical market due to their broad therapeutic applications, cost-effectiveness, and well-established regulatory pathways. With pharmaceutical companies shifting focus toward outsourcing manufacturing and development processes to specialized CDMOs, the market has seen significant expansion. The rising demand for rapid drug development, cost optimization, and flexible manufacturing capabilities has further fueled the need for small molecule CDMOs. Biopharmaceutical companies, ranging from large-scale enterprises to emerging biotech firms, rely on these CDMOs to accelerate time-to-market while ensuring regulatory compliance and production scalability. As the pharmaceutical landscape evolves, small molecule CDMOs are expected to play an even greater role in bridging the gap between drug discovery and commercial production.How Are Technological Advancements Driving Innovation in Small Molecule CDMOs? Exploring Advancements in API Manufacturing and Process Optimization
Technological innovations in synthetic chemistry, process optimization, and automation have significantly enhanced the efficiency of small molecule CDMOs. Continuous manufacturing technologies have emerged as a game-changer, allowing for real-time monitoring, improved yield efficiency, and reduced waste. Advanced analytical techniques, including high-throughput screening and AI-driven drug synthesis modeling, have streamlined compound selection and process development. Additionally, the integration of green chemistry principles has led to the adoption of eco-friendly solvents and enzymatic catalysis techniques, minimizing environmental impact and aligning with global sustainability goals. The use of modular production facilities and single-use bioreactors has further improved operational flexibility, enabling CDMOs to quickly adapt to changing market demands. With increasing investments in R&D and digitalization, small molecule CDMOs are poised to enhance drug development pipelines through innovation, automation, and scalable manufacturing solutions.What Challenges Are Hindering the Growth of the Small Molecule CDMO Market? Addressing Regulatory, Supply Chain, and Cost Pressures
Despite the growing demand for small molecule CDMOs, several challenges persist, including stringent regulatory compliance, supply chain constraints, and cost pressures. The pharmaceutical industry is heavily regulated, with evolving guidelines from agencies such as the U.S. FDA and EMA requiring continuous adaptation to new quality and safety standards. The complexity of global regulatory approvals can delay drug development timelines, adding to the cost burden for both CDMOs and their clients. Supply chain disruptions, exacerbated by global crises and raw material shortages, have impacted active pharmaceutical ingredient (API) sourcing and production capabilities. Additionally, intense competition among CDMOs has led to price pressures, requiring companies to maintain operational efficiency while delivering high-quality services. Addressing these challenges requires strategic investments in regulatory compliance frameworks, diversified supply chains, and cost-effective production methodologies to ensure sustainable growth in the small molecule CDMO sector.What's Driving the Growth of the Small Molecule CDMO Market? Identifying Key Expansion Factors and Industry Trends
The growth in the small molecule CDMO market is driven by several factors, including increasing demand for outsourced pharmaceutical manufacturing, advancements in synthetic chemistry, and the rise of specialty therapeutics. The growing preference for contract-based drug development has encouraged pharmaceutical firms to partner with specialized CDMOs, reducing the need for in-house infrastructure and accelerating drug commercialization. The expansion of precision medicine and targeted small-molecule therapies has further created opportunities for CDMOs specializing in niche drug development. Additionally, the rise of emerging markets and increased pharmaceutical investments in Asia-Pacific and Latin America have opened new growth avenues for CDMOs seeking global expansion. As the pharmaceutical industry continues to evolve with technological advancements and regulatory changes, small molecule CDMOs are expected to remain a cornerstone of drug development, supporting innovation and large-scale production worldwide.Report Scope
The report analyzes the Small Molecule CDMO market, presented in terms of market value (US$). The analysis covers the key segments and geographic regions outlined below:- Segments: Product (Active Pharmaceutical Ingredients, Finished Drug Products); Drug Type (Innovators, Generics); Application (Oncology, Cardiovascular Disease, Central Nervous System Conditions, Autoimmune / Inflammation, Others).
- Geographic Regions/Countries: World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; and Rest of Europe); Asia-Pacific; Rest of World.
Some of the 42 companies featured in this Small Molecule CDMO market report include -
- Aenova Group
- AGC Biologics
- Alcami Corporation
- Apeloa Pharmaceutical
- Aurigene Pharmaceutical Services
- Boehringer Ingelheim
- Cambrex Corporation
- Catalent, Inc.
- CordenPharma International
- CoreRx, Inc.
- Delpharm
- Emergent BioSolutions
- Eurofins Scientific
- Fujifilm Diosynth Biotechnologies
- Hovione
- Labcorp Drug Development
- Lonza Group
- MilliporeSigma
- Patheon (Thermo Fisher Scientific)
- PCI Pharma Services
- Piramal Pharma Solutions
- Recipharm AB
- Samsung Biologics
- Siegfried Holding AG
- Teva Pharmaceutical Industries
- Thermo Fisher Scientific
- Veranova
- Wheeler Bio
- WuXi AppTec
- Zhejiang Huahai Pharmaceutical
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Active Pharmaceutical Ingredients segment, which is expected to reach US$69.8 Billion by 2030 with a CAGR of a 4.7%. The Finished Drug Products segment is also set to grow at 7.9% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $20.6 Billion in 2024, and China, forecasted to grow at an impressive 5.8% CAGR to reach $17.8 Billion by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Small Molecule CDMO Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Small Molecule CDMO Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Small Molecule CDMO Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as BWX Technologies, China National Nuclear Corporation, Copenhagen Atomics, Elysium Industries, Flibe Energy and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Select Competitors (Total 42 Featured):
- Aenova Group
- AGC Biologics
- Alcami Corporation
- Apeloa Pharmaceutical
- Aurigene Pharmaceutical Services
- Boehringer Ingelheim
- Cambrex Corporation
- Catalent, Inc.
- CordenPharma International
- CoreRx, Inc.
- Delpharm
- Emergent BioSolutions
- Eurofins Scientific
- Fujifilm Diosynth Biotechnologies
- Hovione
- Labcorp Drug Development
- Lonza Group
- MilliporeSigma
- Patheon (Thermo Fisher Scientific)
- PCI Pharma Services
- Piramal Pharma Solutions
- Recipharm AB
- Samsung Biologics
- Siegfried Holding AG
- Teva Pharmaceutical Industries
- Thermo Fisher Scientific
- Veranova
- Wheeler Bio
- WuXi AppTec
- Zhejiang Huahai Pharmaceutical
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Aenova Group
- AGC Biologics
- Alcami Corporation
- Apeloa Pharmaceutical
- Aurigene Pharmaceutical Services
- Boehringer Ingelheim
- Cambrex Corporation
- Catalent, Inc.
- CordenPharma International
- CoreRx, Inc.
- Delpharm
- Emergent BioSolutions
- Eurofins Scientific
- Fujifilm Diosynth Biotechnologies
- Hovione
- Labcorp Drug Development
- Lonza Group
- MilliporeSigma
- Patheon (Thermo Fisher Scientific)
- PCI Pharma Services
- Piramal Pharma Solutions
- Recipharm AB
- Samsung Biologics
- Siegfried Holding AG
- Teva Pharmaceutical Industries
- Thermo Fisher Scientific
- Veranova
- Wheeler Bio
- WuXi AppTec
- Zhejiang Huahai Pharmaceutical
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 225 |
| Published | January 2026 |
| Forecast Period | 2024 - 2030 |
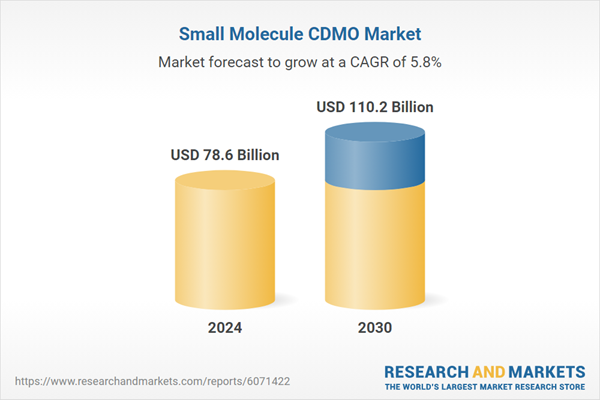
| Estimated Market Value ( USD | $ 78.6 Billion |
| Forecasted Market Value ( USD | $ 110.2 Billion |
| Compound Annual Growth Rate | 5.8% |
| Regions Covered | Global |