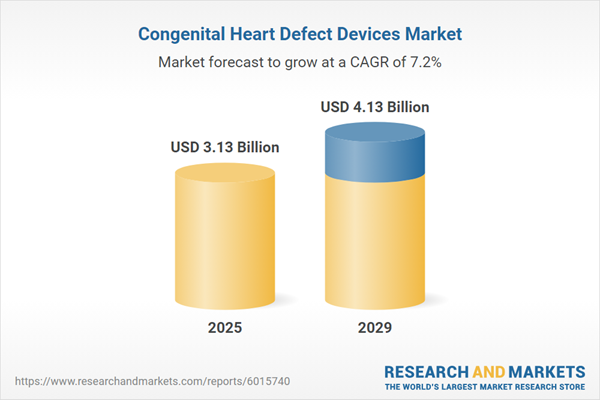
The congenital heart defect devices market size has grown strongly in recent years. It will grow from $2.91 billion in 2024 to $3.13 billion in 2025 at a compound annual growth rate (CAGR) of 7.5%. The growth in the historic period can be attributed to growth in healthcare awareness campaigns, increasing number of heart defect screening programs, growth in medical device regulations, increasing prevalence of congenital heart defect, and increase in the risk of organ failure.
The congenital heart defect devices market size is expected to see strong growth in the next few years. It will grow to $4.13 billion in 2029 at a compound annual growth rate (CAGR) of 7.2%. The growth in the forecast period can be attributed to increasing clinical trials, a surge in the geriatric population, an increasing prevalence of congenital heart defects, rising government support and funding, and an increasing number of specialized medical centers. Major trends in the forecast period include technological advancements, digital health technologies, personalized medicine, 3D printing technology, and wearable devices.
The growth of the congenital heart defect device market is anticipated to be driven by the rising number of minimally invasive procedures. These procedures involve performing surgeries through small incisions or natural body openings, using specialized tools and techniques to minimize trauma and improve recovery. Factors contributing to the increase in minimally invasive procedures include technological advancements, patient preference for less invasive treatments, enhanced surgical techniques, and the advantages of reduced recovery times and complications. Congenital heart defect devices play a crucial role in these procedures by enabling the diagnosis, treatment, and management of heart abnormalities through smaller incisions or catheter-based approaches. For example, according to the American Society of Plastic Surgeons (ASPS), 23,672,269 cosmetic minimally invasive procedures were performed in the United States in 2022, marking a 7% increase from previous years. This rise in minimally invasive procedures is consequently boosting the congenital heart defect device market.
Major players in the congenital heart defect (CHD) devices market are increasingly forming strategic partnerships to develop innovative solutions. These partnerships help companies in the CHD devices sector by combining resources and expertise, enhancing research and development efforts, expanding market reach, and accelerating the commercialization process. Such collaborations foster innovation and contribute to industry growth. For example, in May 2024, SMT (Sahajanand Medical Technologies Pvt Ltd), an India-based manufacturing company, entered into a partnership with HeartX, an India-based MedTech firm, to improve patient care in the cardiovascular sector. Through this collaboration, Sahajanand Medical Technologies (SMT) strengthens its presence in the CHD market by incorporating HeartX's cutting-edge solutions, such as the JOVE VB Stent. This alliance enables SMT to provide advanced CHD devices that improve treatment effectiveness, simplify procedures, and enhance patient outcomes.
In June 2023, Translumina Therapeutics LLP, an India-based cardiovascular device manufacturer, acquired Wellinq Group Company for an undisclosed sum. This acquisition aims to broaden Translumina’s cardiovascular and endovascular solutions portfolio and strengthen its market presence in the Netherlands by integrating Blue Medical Devices’ innovative technologies and expertise. Wellinq Group Company, based in the Netherlands, specializes in developing and manufacturing products for treating congenital heart defects.
Major companies operating in the congenital heart defect devices market are Pfizer Inc., Johnson & Johnson, AstraZeneca plc., Abbott Laboratories, GlaxoSmithKline plc., Medtronic plc., Philips Healthcare, Boston Scientific Corporation, W. L. Gore & Associates Inc., Masimo Corporation, Lepu Medical Technology (Beijing) Co. Ltd., MicroPort Scientific Corporation, Stereotaxis Inc., Berlin Heart GmbH, Ventracor Limited, Syncardia Systems LLC, On-X Life Technologies Inc., Occlutech Holding AG, Sahajanand Medical Technologies Pvt. Ltd., Response Biomedical Corporation, Xeltis AG, Procyrion Inc., Heartstitch Inc., Cardia Inc., OSYPKA Medical GmbH.
North America was the largest region in the congenital heart defect devices market in 2024. Asia-Pacific is expected to be the fastest-growing region in the forecast period. The regions covered in the congenital heart defect devices market report are Asia-Pacific, Western Europe, Eastern Europe, North America, South America, Middle East, Africa. The countries covered in the congenital heart defect devices market report are Australia, Brazil, China, France, Germany, India, Indonesia, Japan, Russia, South Korea, UK, USA, Canada, Italy, Spain.
Congenital heart defect devices are specialized medical instruments and implants designed to address and manage heart abnormalities present from birth. These devices are used to diagnose, treat, and support the heart's function in individuals with congenital heart defects, aiming to restore normal blood flow and enhance cardiac performance.
The primary types of congenital heart defect devices include catheters, pacemakers (single-chamber, dual-chamber, biventricular, and leadless), implantable cardioverter defibrillators, biventricular devices, and implanted cardiac loop recorders. Catheters are slender, flexible tubes inserted into the body for various functions such as diagnostic, therapeutic, or monitoring purposes. These devices are used for different types of congenital defects, including ventricular septal defect, atrioventricular septal defect, tricuspid atresia, truncus arteriosus, and others. They are employed by hospitals, specialty clinics, academic and research institutions, and other healthcare settings.
The congenital heart defect devices market research report is one of a series of new reports that provides congenital heart defect devices market statistics, including congenital heart defect devices industry global market size, regional shares, competitors with a congenital heart defect devices market share, detailed congenital heart defect devices market segments, market trends and opportunities, and any further data you may need to thrive in the Congenital heart defect devices industry. This Congenital heart defect devices market research report delivers a complete perspective of everything you need, with an in-depth analysis of the current and future scenario of the industry.
The congenital heart defect devices market consists of sales of septal occluders, heart valves, patent ductus arteriosus (PDA) devices, and bioprosthetic devices. Values in this market are ‘factory gate’ values, that is the value of goods sold by the manufacturers or creators of the goods, whether to other entities (including downstream manufacturers, wholesalers, distributors and retailers) or directly to end customers. The value of goods in this market includes related services sold by the creators of the goods.
The market value is defined as the revenues that enterprises gain from the sale of goods and/or services within the specified market and geography through sales, grants, or donations in terms of the currency (in USD, unless otherwise specified).
The revenues for a specified geography are consumption values that are revenues generated by organizations in the specified geography within the market, irrespective of where they are produced. It does not include revenues from resales along the supply chain, either further along the supply chain or as part of other products.
This product will be delivered within 3-5 business days.
Table of Contents
Executive Summary
Congenital Heart Defect Devices Global Market Report 2025 provides strategists, marketers and senior management with the critical information they need to assess the market.This report focuses on congenital heart defect devices market which is experiencing strong growth. The report gives a guide to the trends which will be shaping the market over the next ten years and beyond.
Reasons to Purchase:
- Gain a truly global perspective with the most comprehensive report available on this market covering 15 geographies.
- Assess the impact of key macro factors such as conflict, pandemic and recovery, inflation and interest rate environment and the 2nd Trump presidency.
- Create regional and country strategies on the basis of local data and analysis.
- Identify growth segments for investment.
- Outperform competitors using forecast data and the drivers and trends shaping the market.
- Understand customers based on the latest market shares.
- Benchmark performance against key competitors.
- Suitable for supporting your internal and external presentations with reliable high quality data and analysis
- Report will be updated with the latest data and delivered to you along with an Excel data sheet for easy data extraction and analysis.
- All data from the report will also be delivered in an excel dashboard format.
Description
Where is the largest and fastest growing market for congenital heart defect devices ? How does the market relate to the overall economy, demography and other similar markets? What forces will shape the market going forward? The congenital heart defect devices market global report answers all these questions and many more.The report covers market characteristics, size and growth, segmentation, regional and country breakdowns, competitive landscape, market shares, trends and strategies for this market. It traces the market’s historic and forecast market growth by geography.
- The market characteristics section of the report defines and explains the market.
- The market size section gives the market size ($b) covering both the historic growth of the market, and forecasting its development.
- The forecasts are made after considering the major factors currently impacting the market. These include: the Russia-Ukraine war, rising inflation, higher interest rates, and the legacy of the COVID-19 pandemic.
- Market segmentations break down the market into sub markets.
- The regional and country breakdowns section gives an analysis of the market in each geography and the size of the market by geography and compares their historic and forecast growth. It covers the growth trajectory of COVID-19 for all regions, key developed countries and major emerging markets.
- The competitive landscape chapter gives a description of the competitive nature of the market, market shares, and a description of the leading companies. Key financial deals which have shaped the market in recent years are identified.
- The trends and strategies section analyses the shape of the market as it emerges from the crisis and suggests how companies can grow as the market recovers.
Scope
Markets Covered:
1) By Device Type: Catheters; Pacemakers; Single-Chamber Pacemakers; Dual-Chamber Pacemakers; Biventricular Pacemakers; Leadless Pacemakers; Implantable Cardioverter Defibrillators; Biventricular Devices; Implanted Cardiac Loop Recorders; Other Device Types2) By Defect Type: Atrial Septal Defect; Ventricular Septal Defect; Atrioventricular Septal Defect; Tricuspid Atresia; Truncus Arteriosus; Other Defect Types
3) By End user: Hospitals; Specialty Clinics; Academic and Research Institute; Other End Users
Subsegments:
1) By Catheters: Balloon Catheters; Diagnostic Catheters; Guiding Catheters2) By Pacemakers: Single-Chamber Pacemakers; Dual-Chamber Pacemakers; Biventricular Pacemakers; Leadless Pacemakers
3) By Implantable Cardioverter Defibrillators: Single-Chamber Icds; Dual-Chamber Icds; Subcutaneous Icds
4) By Biventricular Devices: Crt-P (Cardiac Resynchronization Therapy Pacemakers); Crt-D (Cardiac Resynchronization Therapy Defibrillators)
5) By Implanted Cardiac Loop Recorders: Traditional Implantable Loop Recorders; Subcutaneous Implantable Loop Recorders
6) By Other Device Types: Transcatheter Heart Valves; Ventricular Assist Devices (VADS)
Key Companies Mentioned: Pfizer Inc.; Johnson & Johnson; AstraZeneca plc.; Abbott Laboratories; GlaxoSmithKline plc.
Countries: Australia; Brazil; China; France; Germany; India; Indonesia; Japan; Russia; South Korea; UK; USA; Canada; Italy; Spain
Regions: Asia-Pacific; Western Europe; Eastern Europe; North America; South America; Middle East; Africa
Time Series: Five years historic and ten years forecast.
Data: Ratios of market size and growth to related markets, GDP proportions, expenditure per capita.
Data Segmentation: Country and regional historic and forecast data, market share of competitors, market segments.
Sourcing and Referencing: Data and analysis throughout the report is sourced using end notes.
Delivery Format: PDF, Word and Excel Data Dashboard.
Companies Mentioned
The major companies featured in this Congenital Heart Defect Devices market report include:- Pfizer Inc.
- Johnson & Johnson
- AstraZeneca plc.
- Abbott Laboratories
- GlaxoSmithKline plc.
- Medtronic plc.
- Philips Healthcare
- Boston Scientific Corporation
- W. L. Gore & Associates Inc.
- Masimo Corporation
- Lepu Medical Technology (Beijing) Co. Ltd.
- MicroPort Scientific Corporation
- Stereotaxis Inc.
- Berlin Heart GmbH
- Ventracor Limited
- Syncardia Systems LLC
- On-X Life Technologies Inc.
- Occlutech Holding AG
- Sahajanand Medical Technologies Pvt. Ltd.
- Response Biomedical Corporation
- Xeltis AG
- Procyrion Inc.
- Heartstitch Inc.
- Cardia Inc.
- OSYPKA Medical GmbH
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 200 |
| Published | April 2025 |
| Forecast Period | 2025 - 2029 |
| Estimated Market Value ( USD | $ 3.13 Billion |
| Forecasted Market Value ( USD | $ 4.13 Billion |
| Compound Annual Growth Rate | 7.2% |
| Regions Covered | Global |
| No. of Companies Mentioned | 26 |