The sudden outbreak of the pandemic had a significant effect on cancer research, which affected the immune checkpoint inhibitors market. For instance, the Lancet article published in December 2021 mentioned that cancer research had been inconsistent, mostly taking place in high-income settings and some middle-income settings such as China and India. Furthermore, the work has largely been undertaken without substantial financial support from major cancer research funders. Thus, the above-mentioned factors are expected to have a significant impact on the growth of the market.
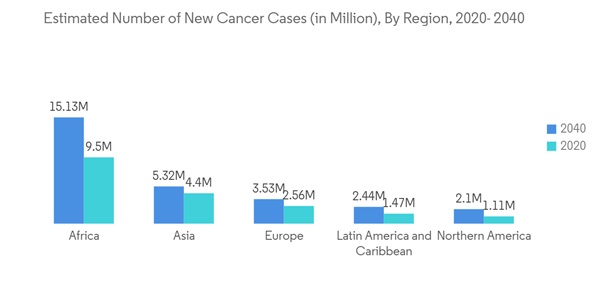
The primary factor attributed to the growth of the market is a steep rise in the global prevalence of various types of cancers, coupled with a steep increase in the geriatric population around the world. The IARC Report published in 2020 estimated the incidence and mortality of 36 cancer in 185 countries. It reported that there were an estimated 19,292,789 new cancer cases diagnosed in 2020, and about 9,958,133 people died due to cancer all over the world. Of the total diagnosed cancer cases, 10,065,305 cases were reported in males, and 9,227,484 cases were reported in females. The incidence of cancer cases in males is expected to reach 15,585,096 by 2040. The number is anticipated to reach 13,302,846 in females by 2040. Such an increasing incidence of cancer among the global population is expected to drive the growth of the market. Thus, the rise in the patient pool, increased awareness among the people, and earlier detection of cancer using technologically advanced screening procedures are likely to boost the immune checkpoint inhibitors market's growth during the forecast period.
However, a higher risk of complications associated with expensive oncology treatment is expected to restrain the growth of the market over the forecast period.
Immune Checkpoint Inhibitors Market Trends
The PD-1 Inhibitors Segment is Expected to Hold the Largest Market Share in the Immune Checkpoint Inhibitors Market
The PD-1 inhibitors segment is expected to account for the largest revenue over the forecast period. The dominance of the segment can be attributed to the increase in research activities, product approvals, and the rise in prescriptions of drugs such as nivolumab and pembrolizumab. For instance, in April 2021, FDA approved nivolumab in combination with chemotherapy for metastatic gastric cancer and esophageal adenocarcinoma. Furthermore, increased adoption and approvals of pembrolizumab (KEYTRUDA) are also expected to drive the growth of the market. For instance, in October 2021, the FDA approved pembrolizumab in combination with chemotherapy, with or without bevacizumab, for patients with persistent, recurrent, or metastatic cervical cancer whose tumors express PD-L1 as determined by an FDA test.In addition, increasing market strategies such as collaborations by the key players for the development of products with low-dose CTLA-4 inhibitors, other non-PD-1, and PD-L1 immune checkpoint inhibitors drugs such as the IDO-1 agents, LAG-3 agents, TIM-3 agents, and CD47/SIRPα agents are likely to propel the market revenue over the forecast period. For instance, in May 2021, Xilo Therapeutics collaborated with Merck to evaluate XTX101, Xilo's engineered tumor-selective Fc- enhanced, anti-CTLA-4 monoclonal antibody product candidate.
Thus, the above-mentioned factors are expected to drive the growth of the segment during the forecast period.
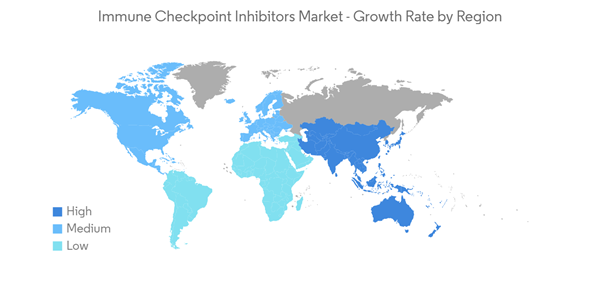
North America is Expected to Dominate the Market During the Forecast Period.
North America is expected to hold a significant share of the market over the forecast period. The dominance is due to the growing prevalence of various chronic diseases like diabetes, skin cancer, urothelial carcinomas, and lung cancers.For instance, according to the ACS, lung cancer was the second most common cancer leading to 135,720 deaths in both men and women in 2020. Additionally, about 13% of all lung cancers were small cell lung cancer (SCLC), and 84% were non-small cell lung cancer (NSCLC) in the United States. This is expected to continue in the future, resulting in higher demand for these products, thereby fueling the market in the region.
Furthermore, the increasing product approvals by the regulatory authorities are expected to contribute to the market's growth. For instance, in August 2021, FDA approved the Glaxosmithkline PD-1 checkpoint inhibitor Jemperli (dostarlimab) for adults with mismatch repair-deficient recurrent or advanced endometrial cancer. Jemperli is an anti-PD-1 antibody that binds to the PD-1 receptor and blocks its interaction with the PD-1 ligands PD-L1 and PD-L2.
Moreover, the significant share of market revenue in the North American region can be attributed to the presence of major key players and huge investment in R&D activities to develop new products with lesser side effects, along with initiatives by the government to encourage innovations to decrease the growing disease burden. This is likely to provide more opportunities for the growth of the immune checkpoint inhibitors market during the forecast period.
Immune Checkpoint Inhibitors Market Competitor Analysis
The immune checkpoint inhibitors market is moderately competitive and consists of several major players. Some of the companies are expanding their market position by adopting various strategies such as acquisitions and mergers, and research collaboration, while others are investing in clinical trials to extend the treatment of other indications with the existing drugs to address the unmet challenges of the disease burden driving the market share. A few of the major players currently dominating the industry in terms of revenue are Bristol‑Myers Squibb Company, AstraZeneca PLC, F. Hoffmann-La Roche AG, Regeneron Pharmaceuticals Inc., and Merck & Co. Inc.Additional benefits of purchasing the report:
- The market estimate (ME) sheet in Excel format
- 3 months of analyst support
This product will be delivered within 2 business days.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Bristol-Myers Squibb Company
- Merck & Co.
- F. Hoffmann-La Roche AG
- Regeneron Pharmaceuticals Inc.
- AstraZeneca PLC
- Eli Lilly and Company
- Sanofi
- BeiGene Ltd
- Shanghai Jhunsi Biosciences Ltd
- GlaxoSmithKline PLC
- Immutep Ltd