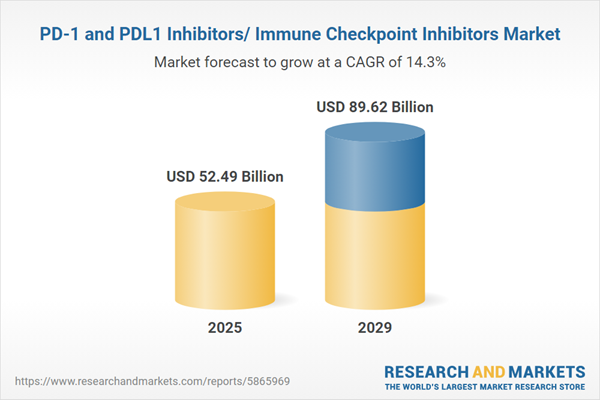
The PD-1 and PDL1 inhibitors/ immune checkpoint inhibitors market size is expected to see rapid growth in the next few years. It will grow to $89.62 billion in 2029 at a compound annual growth rate (CAGR) of 14.3%. The growth in the forecast period can be attributed to expanding indications, biomarker research and personalized medicine, clinical trials and research collaborations, and global healthcare infrastructure development. Major trends in the forecast period include a focus on patient access and affordability, global clinical trial collaborations, immunotherapy combinations, patient-centric approaches, regulatory updates, and approvals.
The forecast of 14.3% growth over the next five years reflects a modest reduction of 0.4% from the previous estimate for this market. This reduction is primarily due to the impact of tariffs between the US and other countries. Tariff barriers are expected to hamper U.S. oncology centers by increasing the cost of programmed cell death protein 1 inhibitors developed in the UK and Japan, thereby limiting immunotherapy access for cancer patients and raising precision medicine expenditures. The effect will also be felt more widely due to reciprocal tariffs and the negative effect on the global economy and trade due to increased trade tensions and restrictions.
The projected growth in the PD-1 and PD-L1 Inhibitors, also known as Immune Checkpoint Inhibitors, market is heavily influenced by the escalating prevalence of cancer cases. Cancer, characterized by uncontrolled cell growth and dissemination, has seen a noticeable increase in cases over recent years. PD-1 and PD-L1 inhibitors play a crucial role in treating cancer by leveraging the body's immune system to combat cancer cells effectively. For example, as per the European Union Science Hub's findings in October 2023, the preceding two years witnessed a 2.3% rise in new cancer cases, reaching 2.74 million, alongside a 2.4% increase in cancer-related deaths. This surge in cancer incidence significantly contributes to the growth anticipated in the PD-1 and PD-L1 Inhibitors market.
The anticipated growth in the PD-1 and PD-L1 Inhibitors market is closely tied to the increasing emphasis on personalized medicine. This advanced healthcare approach considers unique patient factors such as genetics, behaviors, and environmental influences in treatment strategies. In the context of PD-1 and PD-L1 inhibitors, personalized medicine involves tailoring cancer therapies to optimize efficacy and minimize potential adverse effects based on individual patient characteristics and biomarker expressions. Notably, the Personalized Medicine Coalition's data from February 2023 revealed that personalized medications accounted for 34% of newly approved pharmaceuticals by the US FDA in 2022, showcasing a sustained upward trend. This growing focus on personalized medicine significantly drives the PD-1 and PD-L1 Inhibitors market forward.
Product innovation has emerged as a pivotal trend shaping the landscape of PD-1 and PD-L1 Inhibitors, or immune checkpoint inhibitors. Leading market players are strategically channeling efforts towards developing innovative products to bolster their market presence. For instance, Bristol-Myers Squibb's successful FDA approval for Opdualag in March 2022 marked a breakthrough - a fixed-dose combination of nivolumab (Opdivo) and relatlimab, the latter being a LAG-3 inhibitor. Opdualag, the first LAG-3 inhibitor approved by the US FDA for treating metastatic melanoma, aims to augment immune responses against cancer cells, showcasing a substantial leap in product innovation within this sector.
Strategic investments play a crucial role in shaping the landscape of the PD-1 and PD-L1 Inhibitors market. Major companies operating in this domain are leveraging strategic initiatives to fortify their positions. For instance, Regeneron Pharmaceuticals, Inc.'s investment in June 2022 involved acquiring Sanofi's share in the Regeneron and Sanofi partnership concerning Libtayo (cemiplimab). This strategic move conferred Regeneron exclusive rights to develop, market, and manufacture Libtayo globally. Valued at $900 million, plus royalties and potential future milestone payments, this investment aimed to bolster Regeneron's foothold in the market. Libtayo, a first-in-class PD-1 inhibitor developed using Regeneron's VelocImmune technology, gained approval from the FDA and numerous global regulatory bodies, solidifying its position as a standard treatment for certain advanced non-small cell lung cancer, cutaneous squamous cell carcinoma, and basal cell carcinoma cases.
In June 2023, Coherus BioSciences, a leading U.S.-based biopharmaceutical company specializing in cancer therapeutics, acquired Surface Oncology for $65 million. This acquisition enhances Coherus' portfolio by incorporating innovative immuno-oncology therapies, including Surface's IL-27-targeted antibody (SRF388) and CCR8-targeted antibody (SRF114). With this acquisition, Coherus can now provide a diverse range of clinical-stage immunotherapies designed to boost the immune response against tumors, including applications for lung and liver cancer. Surface Oncology is a U.S.-based firm engaged in developing PD-1 and PD-L1 inhibitors, which are essential elements of immune checkpoint therapy for cancer treatment.
Major companies operating in the PD-1 and PDL1 inhibitors/ immune checkpoint inhibitors market include Bristol-Myers Squibb Company, Merck and Company, F. Hoffmann-La Roche AG, Sanofi S.A., Amgen Inc., Gilead Sciences Inc., AstraZeneca plc, Novartis AG, Pfizer Inc., GlaxoSmithKline Plc, Regeneron Pharmaceuticals Inc., BeiGene Ltd, Shanghai Jhunsi Biosciences Ltd., Akeso Inc., Alphamab Oncology, Eli Lilly and Company, Boehringer Ingelheim, Xencor Inc., Taiga Biotechnologies Inc., Jiangsu Alphamab Biopharmaceuticals Co. Ltd, Dr. Reddy's Laboratories Ltd., Laekna Therapeutics, Genentech Inc., Tracon Pharmaceuticals Inc., Celgene, Hangzhou Sumgen Co. Ltd, Agenus Inc., BioNTech SE, Celldex Therapeutics Inc., CytomX Therapeutics Inc.
North America was the largest region in the PD-1 and PDL1 inhibitors market in 2024. The regions covered in the pd-1 and pdl1 inhibitors/ immune checkpoint inhibitors market report are Asia-Pacific, Western Europe, Eastern Europe, North America, South America, Middle East, Africa. The countries covered in the pd-1 and pdl1 inhibitors/ immune checkpoint inhibitors market report are Australia, Brazil, China, France, Germany, India, Indonesia, Japan, Russia, South Korea, UK, USA, Canada, Italy, Spain.
Note that the outlook for this market is being affected by rapid changes in trade relations and tariffs globally. The report will be updated prior to delivery to reflect the latest status, including revised forecasts and quantified impact analysis. The report’s Recommendations and Conclusions sections will be updated to give strategies for entities dealing with the fast-moving international environment.
The sudden escalation of U.S. tariffs and the resulting trade tensions in spring 2025 are having a significant impact on the pharmaceutical sector. Companies are grappling with higher costs on imported active pharmaceutical ingredients (APIs), glass vials, and laboratory equipment - many of which have limited alternative sources. Generic drug manufacturers, already operating with minimal profit margins, are particularly affected, with some scaling back production of low-margin medications. Biotech firms are also experiencing delays in clinical trials due to shortages of specialized reagents linked to tariffs. In response, the industry is shifting API production to regions like India and Europe, building up inventory reserves, and advocating for tariff exemptions on essential medicines.
These immunotherapy drugs are available through various channels including hospital pharmacies, retail pharmacies, and online platforms. They are predominantly utilized within hospital settings, specialty clinics, as well as academic and research institutions, constituting integral elements in comprehensive cancer care and research endeavors.
PD-1 and PDL1 inhibitors, also known as immune checkpoint inhibitors, belong to the class of immunotherapy drugs employed in cancer treatment. They function by directing the immune system to identify and combat cancer cells effectively. Widely applied across various cancer conditions, these therapies serve as vital components in cancer treatment protocols.
Key products within the PD-1 and PDL1 inhibitor category include nivolumab, pembrolizumab, atezolizumab, avelumab, and durvalumab. Nivolumab, specifically a PD-1 inhibitor, operates by impeding the PD-1 receptor present on T cells, thereby thwarting cancer cells' ability to evade immune system attacks. These inhibitors find application in treating a spectrum of cancers such as lung cancer, bladder cancer, melanoma, Hodgkin lymphoma, colorectal cancer, among others.
The PD-1 and PDL1 Inhibitors/ Immune checkpoint inhibitors market consists of sales of various inhibitors such as cemiplimab, sintilimab, and camrelizumab. Values in this market are ‘factory gate’ values, that is the value of goods sold by the manufacturers or creators of the goods, whether to other entities (including downstream manufacturers, wholesalers, distributors, and retailers) or directly to end customers. The value of goods in this market includes related services sold by the creators of the goods.
The market value is defined as the revenues that enterprises gain from the sale of goods and/or services within the specified market and geography through sales, grants, or donations in terms of the currency (in USD, unless otherwise specified).
The revenues for a specified geography are consumption values that are revenues generated by organizations in the specified geography within the market, irrespective of where they are produced. It does not include revenues from resales along the supply chain, either further along the supply chain or as part of other products.
This product will be delivered within 1-3 business days.
Table of Contents
Executive Summary
PD-1 and PDL1 Inhibitors/ Immune Checkpoint Inhibitors Global Market Report 2025 provides strategists, marketers and senior management with the critical information they need to assess the market.This report focuses on pd-1 and pdl1 inhibitors/ immune checkpoint inhibitors market which is experiencing strong growth. The report gives a guide to the trends which will be shaping the market over the next ten years and beyond.
Reasons to Purchase:
- Gain a truly global perspective with the most comprehensive report available on this market covering 15 geographies.
- Assess the impact of key macro factors such as geopolitical conflicts, trade policies and tariffs, post-pandemic supply chain realignment, inflation and interest rate fluctuations, and evolving regulatory landscapes.
- Create regional and country strategies on the basis of local data and analysis.
- Identify growth segments for investment.
- Outperform competitors using forecast data and the drivers and trends shaping the market.
- Understand customers based on the latest market shares.
- Benchmark performance against key competitors.
- Suitable for supporting your internal and external presentations with reliable high quality data and analysis
- Report will be updated with the latest data and delivered to you along with an Excel data sheet for easy data extraction and analysis.
- All data from the report will also be delivered in an excel dashboard format.
Description
Where is the largest and fastest growing market for pd-1 and pdl1 inhibitors/ immune checkpoint inhibitors? How does the market relate to the overall economy, demography and other similar markets? What forces will shape the market going forward, including technological disruption, regulatory shifts, and changing consumer preferences? The pd-1 and pdl1 inhibitors/ immune checkpoint inhibitors market global report answers all these questions and many more.The report covers market characteristics, size and growth, segmentation, regional and country breakdowns, competitive landscape, market shares, trends and strategies for this market. It traces the market’s historic and forecast market growth by geography.
- The market characteristics section of the report defines and explains the market.
- The market size section gives the market size ($b) covering both the historic growth of the market, and forecasting its development.
- The forecasts are made after considering the major factors currently impacting the market. These include: the technological advancements such as AI and automation, Russia-Ukraine war, trade tariffs (government-imposed import/export duties), elevated inflation and interest rates.
- Market segmentations break down the market into sub markets.
- The regional and country breakdowns section gives an analysis of the market in each geography and the size of the market by geography and compares their historic and forecast growth.
- The competitive landscape chapter gives a description of the competitive nature of the market, market shares, and a description of the leading companies. Key financial deals which have shaped the market in recent years are identified.
- The trends and strategies section analyses the shape of the market as it emerges from the crisis and suggests how companies can grow as the market recovers.
Scope
Markets Covered:
1) By Product: Nivolumab; Pembrolizumab; Atezolizumab; Avelumab; Durvalumab2) By Distribution Channel: Hospital pharmacies; Retail pharmacies; Online pharmacies
3) By Application: Lung Cancer; Bladder Cancer; Melanoma; Hodgkin Lymphoma; Colorectal Cancer; Other Applications
4) By End-Users : Hospitals; Specialty Clinics; Academic and Research Institutions
Subsegments:
1) By Nivolumab: Monotherapy; Combination Therapy2) By Pembrolizumab: Monotherapy; Combination Therapy
3) By Atezolizumab: Monotherapy; Combination Therapy
4) By Avelumab: Monotherapy; Combination Therapy
5) By Durvalumab: Monotherapy; Combination Therapy
Companies Mentioned: Bristol-Myers Squibb Company; Merck and Company; F. Hoffmann-La Roche AG; Sanofi S.A.; Amgen Inc.; Gilead Sciences Inc.; AstraZeneca plc; Novartis AG; Pfizer Inc.; GlaxoSmithKline Plc; Regeneron Pharmaceuticals Inc.; BeiGene Ltd; Shanghai Jhunsi Biosciences Ltd.; Akeso Inc.; Alphamab Oncology; Eli Lilly and Company; Boehringer Ingelheim; Xencor Inc.; Taiga Biotechnologies Inc.; Jiangsu Alphamab Biopharmaceuticals Co. Ltd; Dr. Reddy's Laboratories Ltd.; Laekna Therapeutics; Genentech Inc.; Tracon Pharmaceuticals Inc.; Celgene; Hangzhou Sumgen Co. Ltd; Agenus Inc.; BioNTech SE; Celldex Therapeutics Inc.; CytomX Therapeutics Inc.
Countries: Australia; Brazil; China; France; Germany; India; Indonesia; Japan; Russia; South Korea; UK; USA; Canada; Italy; Spain
Regions: Asia-Pacific; Western Europe; Eastern Europe; North America; South America; Middle East; Africa
Time Series: Five years historic and ten years forecast.
Data: Ratios of market size and growth to related markets, GDP proportions, expenditure per capita.
Data Segmentation: Country and regional historic and forecast data, market share of competitors, market segments.
Sourcing and Referencing: Data and analysis throughout the report is sourced using end notes.
Delivery Format: PDF, Word and Excel Data Dashboard.
Companies Mentioned
The companies featured in this PD-1 and PDL1 Inhibitors/ Immune Checkpoint Inhibitors market report include:- Bristol-Myers Squibb Company
- Merck and Company
- F. Hoffmann-La Roche AG
- Sanofi S.A.
- Amgen Inc.
- Gilead Sciences Inc.
- AstraZeneca plc
- Novartis AG
- Pfizer Inc.
- GlaxoSmithKline Plc
- Regeneron Pharmaceuticals Inc.
- BeiGene Ltd
- Shanghai Jhunsi Biosciences Ltd.
- Akeso Inc.
- Alphamab Oncology
- Eli Lilly and Company
- Boehringer Ingelheim
- Xencor Inc.
- Taiga Biotechnologies Inc.
- Jiangsu Alphamab Biopharmaceuticals Co. Ltd
- Dr. Reddy's Laboratories Ltd.
- Laekna Therapeutics
- Genentech Inc.
- Tracon Pharmaceuticals Inc.
- Celgene
- Hangzhou Sumgen Co. Ltd
- Agenus Inc.
- BioNTech SE
- Celldex Therapeutics Inc.
- CytomX Therapeutics Inc.
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 250 |
| Published | September 2025 |
| Forecast Period | 2025 - 2029 |
| Estimated Market Value ( USD | $ 52.49 Billion |
| Forecasted Market Value ( USD | $ 89.62 Billion |
| Compound Annual Growth Rate | 14.3% |
| Regions Covered | Global |
| No. of Companies Mentioned | 30 |