Surge in launch of novel cardiac mapping device is predicted to boost the market growth during the forecast period. Cardiac mapping involves the assessment of the heart's electrical activity, aiding physicians in diagnosing cardiovascular diseases and making predictions about heart health. These devices record the electrical signals transmitted by a specialized catheter and store patient data. Electrophysiologists commonly rely on this highly effective tool to pinpoint the areas within the heart where arrhythmias or irregular heart rhythms are occurring. Therefore, with the rising prevalence of cardiac complications, there is an anticipated increase in the demand for cardiac mapping. For instance, in September 2022, Biosense Webster, Inc., a division of Johnson & Johnson MedTech, has introduced the OCTARAY Mapping Catheter featuring TRUEref Technology, which is powered by the CARTO 3 Version 7 System. This catheter is tailored for the mapping of cardiac arrhythmias like Atrial Fibrillation (AFib). Equipped with eight splines that provide improved electrode spacing choices, the OCTARAY Mapping Catheter enables quicker and more efficient mapping compared to the PENTARAY NAV ECO Mapping Catheter.
By type, contact cardiac mapping was the highest revenue-grossing segment in the global cardiac mapping market in 2023 owing to its benefits include the ability to locate mapping & ablation catheters precisely in a three-dimensional environment, increasing demand for diagnosis of complex arrhythmias, and rising approvals by regulatory bodies. For instance, in January 2022, Abbott has introduced the EnSite X EP System featuring EnSite Omnipolar Technology (OT), an innovative cardiac mapping system designed to improve the management of cardiac arrhythmias. This system has received approval from the U.S. Food and Drug Administration and is now accessible in both the United States and Europe. Additionally, non-contact cardiac mapping systems is predicted to grow at fastest CAGR during the forecast period owing to the growing launch of innovative products in diagnosing disorders and increasing technological advancements.
By indication, atrial fibrillation was the highest revenue-grossing segment in the global cardiac mapping market in 2023 owing to the increasing prevalence of atrial fibrillation, growing adoption of cardiac mapping in atrial fibrillation therapies, rising demand for the detection of triggers & substrates to optimize an ablation technique, and surge in launch of new products. For instance, in April 2022, At the 2022 European Heart Rhythm Association annual meeting, Philips unveiled its most recent KODEX-EPD cardiac mapping and navigation system. Additionally, Atrial Flutter is predicted to grow at fastest CAGR during the forecast period owing to the growing burden of atrial flutter, rising demand for early diagnosis, and increasing technological developments.
By end-user, hospitals & clinics was the highest revenue-grossing segment in the global cardiac mapping market in 2023 owing to the wide availability of modern cardiovascular therapy tools in hospitals, growing prevalence of cardiovascular disorders, rising incorporation of advanced technologies for cardiac mapping techniques, and increasing regulatory approvals of novel deivces. For instance, in February 2022, Vektor Medical has disclosed that UC San Diego Health will be the inaugural hospital system globally to provide its newly FDA-approved vMap. vMap is a pioneering tool that relies solely on 12-lead ECG data to pinpoint potential arrhythmia sources within the heart in under three minutes. The company plans to introduce this technology gradually, starting at specific cardiovascular centers of excellence throughout the country over the next year, before making it widely available. Additionally, ambulatory surgical centers (ASC) is predicted to grow at fastest CAGR during the forecast period owing to the expansion of outpatient cardiac procedures, increasing favorable reimbursement, a rise in covered treatments & improved patient outcomes, and surge in adoption of advanced technologies.
North America region is anticipated for the highest revenue share during the forecast period owing to the rising prevalence of cardiac diseases, growing healthcare expenditures, surge in product launches, increasing product approvals for cardiac mapping by regulatory bodies. For instance, in March 2023, Medtronic has revealed that it obtained the CE Mark for the Affera Mapping and Ablation System, comprising the Sphere-9 Catheter and the Affera Prism-1 Mapping Software. This complete system represents a groundbreaking development in electrophysiology, as it seamlessly combines the Sphere-9 pulsed field ablation (PFA), radiofrequency (RF), and high-density (HD) mapping catheter. This innovative technology is designed for mapping and ablating atrial arrhythmias, providing instant feedback through its user-friendly mapping and navigation software. Additionally, Europe region is predicted to grow at fastest CAGR during the forecast period owing to the rising technological advancements, growing prevalence of cardiac disorders, and increasing mergers & acquisitions within market players. For instance, in August 2022, Medtronic plc, a prominent player in healthcare technology worldwide, has successfully finalized its acquisition of Affera, Inc. This acquisition marks an expansion of the company's cardiac ablation offerings, now incorporating a comprehensive cardiac mapping and navigation platform that includes a unique and fully integrated diagnostic system, focal pulsed field ablation, and radiofrequency ablation solution.
Segmentation: Cardiac Mapping Market Report 2023 - 2034
Cardiac Mapping Market Analysis & Forecast by Type 2023 - 2034 (Revenue USD Bn)
- Non-Contact Cardiac Mapping Systems
- Contact Cardiac Mapping Systems
- Basket Catheter Mapping
- Electroanatomical Mapping
- Traditional Endocardial Catheter Mapping
Cardiac Mapping Market Analysis & Forecast by Indication 2023 - 2034 (Revenue USD Bn)
- Atrial Flutter
- Atrial Fibrillation
- Other
Cardiac Mapping Market Analysis & Forecast by End-user 2023 - 2034 (Revenue USD Bn)
- Diagnostic Centers
- Hospitals & Clinics
- Ambulatory Surgical Centers
Cardiac Mapping Market Analysis & Forecast by Region 2023 - 2034 (Revenue USD Bn)
- North America
- U.S.
- Canada
- Europe
- Germany
- France
- UK
- Spain
- Italy
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- Australia
- South Korea
- Rest of APAC
- Latin America
- Brazil
- Mexico
- Argentina
- Rest of LATAM
- Middle East & Africa
- South Africa
- GCC
- Rest of MEA
Table of Contents
Companies Mentioned
- Johnson and Johnson (Biosense Webster)
- Koninklijke Philips
- Abbott Laboratories
- Acutus Medical Inc.
- BioSig Technologies
- EPMap Systems
- Kardium Inc.
- Medtronic Plc.
- Lepu Medical Technology
- Boston Bioscientific Corporation
- APN Healthcare
- MicroPort Scientific Corporation
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 200 |
| Published | January 2024 |
| Forecast Period | 2023 - 2034 |
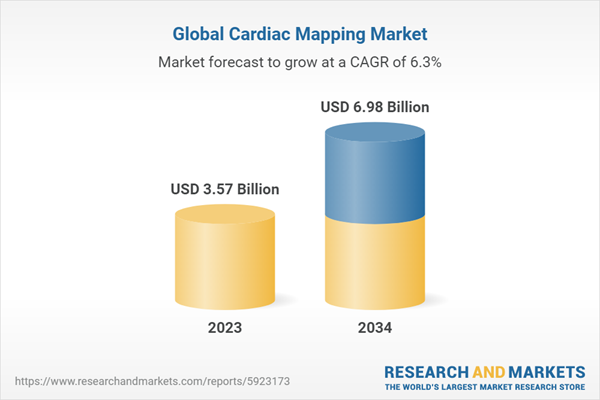
| Estimated Market Value ( USD | $ 3.57 Billion |
| Forecasted Market Value ( USD | $ 6.98 Billion |
| Compound Annual Growth Rate | 6.2% |
| Regions Covered | Global |
| No. of Companies Mentioned | 12 |