Speak directly to the analyst to clarify any post sales queries you may have.
10% Free customizationThis report comes with 10% free customization, enabling you to add data that meets your specific business needs.
As the pharmaceutical and biotechnology industries expand, the need for comprehensive cardiac safety testing for new drugs and therapies has surged. Regulatory requirements and the focus on patient safety during clinical trials also fuel market growth. Advancements in cardiac safety testing technologies, including electrocardiogram (ECG) and other non-invasive monitoring systems, are further enhancing the efficiency of these services. The growing aging population, coupled with the adoption of personalized medicine, continues to drive demand for specialized cardiac safety assessments, ensuring the market's steady expansion.
Key Market Drivers
Increasing Prevalence of Cardiovascular Diseases (CVDs)
The rise in cardiovascular diseases (CVDs) worldwide significantly influences the demand for cardiac safety services. CVDs, including coronary artery disease, heart attacks, heart failure, arrhythmias, and strokes, are among the most common and deadly health conditions globally. The World Health Organization (WHO) reports that CVDs are responsible for more than 30% of all deaths worldwide. Factors contributing to this increase include urbanization, aging populations, and unhealthy lifestyle choices such as poor diet, smoking, sedentary behavior, and stress.This growing burden of cardiovascular conditions calls for enhanced research, drug development, and therapeutic interventions, all of which must undergo thorough safety assessments, particularly concerning the heart. According to Centers for Disease Control and Prevention, Heart disease is the leading cause of death for both men and women, as well as for individuals across most racial and ethnic groups. Every 33 seconds, someone dies from cardiovascular disease. In 2022 alone, heart disease was responsible for 702,880 deaths, accounting for roughly one in every five deaths.
As pharmaceutical and biotechnology companies innovate with new drugs targeting CVDs, cardiac safety testing becomes crucial. These tests aim to ensure that treatments do not exacerbate existing heart conditions or cause dangerous side effects like arrhythmias, heart failure, or other cardiac complications. This demand for safe and effective treatments is driving the global cardiac safety services market, as these services are required to assess the risks associated with new therapies and drugs. With cardiovascular disease affecting millions of people worldwide, there is a consistent and increasing need for cardiac safety services that ensure patient health during drug development and clinical trials.
Aging Global Population
The aging population is a significant factor contributing to the growth of the cardiac safety services market. As life expectancy increases, the number of elderly individuals who are susceptible to cardiovascular diseases grows, creating a higher demand for healthcare services, including cardiac safety monitoring. According to the United Nations, the global population of people aged 60 years or older is expected to reach 2.1 billion by 2050, up from 1 billion in 2020. Older adults are more likely to develop CVDs, including hypertension, heart failure, and arrhythmias, which increases their need for cardiac care and monitoring.As older populations tend to use multiple medications, they are at a higher risk for adverse drug reactions, including cardiac complications. This creates a demand for comprehensive cardiac safety assessments during drug trials and post-market surveillance to ensure that new and existing treatments do not pose excessive risk to these vulnerable patients. Elderly patients often have complex co-morbidities, which necessitate personalized approaches to drug development and safety testing. As a result, the aging global population directly contributes to the growing need for cardiac safety services to ensure that therapeutic interventions are safe and effective for elderly patients.
Focus on Personalized Medicine
The growing emphasis on personalized medicine is another driver for the cardiac safety services market. Personalized medicine aims to tailor medical treatments to individual patients based on their genetic makeup, lifestyle, and other factors. In cardiovascular care, this approach involves developing treatments that are specific to an individual’s unique risk profile, including their susceptibility to heart disease and how they might respond to various drugs. In November 2023, iRhythm Technologies, Inc. launched its latest Zio monitor along with the enhanced Zio (LTCM) Long-term Continuous Monitoring service in the U.S. This cutting-edge technology aims to elevate cardiac monitoring by providing more precise and detailed long-term assessments.As personalized treatments become more common, there is a greater need for precise and targeted cardiac safety assessments. For example, genetic testing and biomarkers are used to predict how patients will respond to specific cardiovascular medications. Cardiac safety services play an essential role in ensuring that these treatments are safe and do not cause adverse cardiac events. Personalized medicine often requires more sophisticated testing methods and more comprehensive safety monitoring to address the specific needs of individual patients, further driving the market for cardiac safety services. The integration of these services into the development of personalized therapies ensures that new treatments are not only effective but also safe for the intended patient population.
Increase in Clinical Trials for Cardiovascular Drugs
The expansion of clinical trials focused on cardiovascular drugs is another major driver of the global cardiac safety services market. As the demand for new and innovative cardiovascular treatments grows, more clinical trials are being conducted worldwide to test the safety and efficacy of these drugs. Clinical trials are a crucial step in the drug development process, and cardiovascular drugs require specialized safety evaluations due to the risks they pose to heart health.During clinical trials, continuous cardiac monitoring is essential to identify any adverse effects that may occur due to the drug. Cardiac safety services provide the necessary expertise to monitor participants for any signs of cardiac complications such as arrhythmias, hypertension, and QT interval prolongation. As more pharmaceutical companies and research institutions initiate large-scale clinical trials for cardiovascular drugs, the need for specialized cardiac safety services continues to grow. This is especially true for early-phase trials, where patient safety is paramount. Thus, the increasing number of clinical trials for cardiovascular drugs directly contributes to the market’s expansion.
Rising Patient Awareness and Preventive Healthcare Initiatives
There is a growing awareness among patients about the importance of heart health, which has contributed to an increased focus on preventive healthcare. People are becoming more conscious of the risks associated with cardiovascular diseases, leading to greater demand for regular checkups, diagnostic testing, and safety monitoring. With the advent of health-focused apps and wearable technologies that track heart rate, blood pressure, and ECGs, individuals are becoming more proactive in managing their cardiovascular health. In February 2023, GE HealthCare announced its plans to acquire Caption Health, a company specializing in AI software for cardiac imaging guidance. The technology developed by Caption Health is designed to simplify and accelerate ultrasound exams, allowing a broader range of healthcare professionals to conduct basic echocardiography tests.This shift toward preventive care has created a heightened demand for cardiac safety services, as both patients and healthcare providers recognize the importance of early detection and monitoring. Healthcare systems are increasingly focusing on proactive management of heart health, encouraging patients to undergo routine cardiac evaluations, particularly when starting new medications or undergoing high-risk procedures. The increasing emphasis on preventive healthcare and patient education ensures a continued demand for cardiac safety services, as people seek to maintain heart health and prevent adverse events through early intervention.
Key Market Challenges
High Costs of Cardiac Safety Testing
One of the primary challenges in the cardiac safety services market is the high cost associated with advanced cardiac safety testing. The procedures and technologies required for thorough heart monitoring, such as electrocardiograms (ECGs), echocardiograms, and advanced imaging techniques, are often expensive. The need for specialized equipment, highly trained personnel, and regulatory compliance adds to the overall cost.For pharmaceutical companies, these expenses can be significant, especially for smaller organizations or those in the early stages of drug development. The high costs can lead to financial constraints, limiting the number of drugs that undergo comprehensive cardiac safety evaluations, and may discourage innovation in drug development for cardiovascular diseases. Smaller biotech firms may find it challenging to secure funding for conducting such extensive testing, potentially affecting the pace and breadth of clinical trials.
Regulatory and Compliance Complexities
Navigating the complex regulatory landscape is another significant challenge for the cardiac safety services market. Different countries and regions have varying guidelines and regulatory standards for cardiac safety testing, making it difficult for pharmaceutical companies and service providers to maintain consistency across global trials. For example, the FDA, EMA, and other regulatory bodies each have their own specific requirements when it comes to conducting clinical trials and ensuring patient safety, including cardiac monitoring. This creates an administrative burden for organizations involved in drug development, as they must ensure compliance with multiple sets of rules. Any failure to meet these requirements can result in delays, regulatory fines, or even the rejection of drug approvals. Ensuring consistent and standardized cardiac safety assessments across different regions is challenging due to disparities in infrastructure and resources available in different healthcare systems.Limited Availability of Trained Professionals
The shortage of healthcare professionals and specialists with expertise in cardiac safety testing presents a significant challenge. Cardiac safety testing requires skilled personnel, including cardiologists, electrophysiologists, and clinical researchers, who are proficient in using advanced diagnostic tools and interpreting the results. However, there is a global shortage of such specialized professionals, particularly in developing regions.This shortage can lead to delays in clinical trials, lower-quality testing, and difficulties in maintaining the high standards necessary for ensuring patient safety. There is an ongoing challenge of training new professionals to keep up with the rapidly evolving technologies in the field of cardiac safety, including the integration of AI and advanced data analytics. Without a sufficient workforce of qualified professionals, the growth of the cardiac safety services market could be hindered, as the demand for expertise continues to outpace the supply of skilled labor.
Key Market Trends
Technological Advancements in Cardiac Safety Testing
Technological innovations in cardiac safety testing have significantly impacted the growth of the global cardiac safety services market. Advances in diagnostic tools such as electrocardiograms (ECGs), echocardiograms, ambulatory blood pressure monitors, and non-invasive imaging technologies are revolutionizing cardiac safety testing. These technologies allow for more accurate, efficient, and real-time monitoring of heart function during drug trials, enabling researchers to identify potential adverse cardiac events early in the testing process.The integration of artificial intelligence (AI) and machine learning in analyzing ECG data and other cardiac biomarkers is enhancing the accuracy of safety evaluations. AI algorithms can quickly analyze large datasets, identify patterns, and predict risks that might otherwise go unnoticed. This has led to more precise and reliable results in drug safety assessments. As the capabilities of cardiac testing technologies continue to improve, the demand for these services has grown, driving market expansion. The ability to detect early signs of cardiac risk without invasive procedures is increasing patient comfort and compliance, further supporting the market's growth.
Growth of the Pharmaceutical and Biotech Industries
The continuous growth of the pharmaceutical and biotechnology sectors is a key driver of the cardiac safety services market. As these industries expand and focus on the development of novel therapies, particularly for cardiovascular conditions, there is a growing need for specialized safety assessments. Many pharmaceutical companies are investing heavily in cardiovascular drug development, with the aim of addressing the rising global incidence of CVDs.New drug candidates and treatments are often tested in preclinical and clinical settings to assess their efficacy and safety. For drugs targeting the cardiovascular system, cardiac safety is a particularly crucial area of concern. The rise in the number of cardiovascular drug development programs requires increased demand for cardiac safety services, including clinical trials, safety monitoring, and post-market surveillance. These services ensure that potential new drugs do not introduce risks such as arrhythmias, QT interval prolongation, or other harmful cardiac effects, which can be life-threatening. Thus, the expansion of pharmaceutical and biotech industries plays a direct role in driving the need for these services.
Segmental Insights
Service Insights
Based on the service, ECG/Holter Monitors was the fastest-growing segment in the cardiac safety services market, driven by their widespread use, critical role in cardiac safety testing, and increasing demand for non-invasive monitoring solutions. ECGs and Holter monitors are essential tools in clinical trials and routine cardiovascular care, providing real-time insights into the heart's electrical activity. They play a pivotal role in detecting potential arrhythmias, QT prolongation, and other cardiovascular risks that can arise during drug development or treatment.The rapid growth of ECG/Holter monitors is fueled by their non-invasive nature and ease of use, making them attractive for both routine patient monitoring and drug trials. In drug development, these devices are vital for assessing potential cardiac risks of new drugs, particularly for compounds targeting the cardiovascular system, as QT prolongation and arrhythmias are common and serious side effects. Regulatory agencies, such as the FDA and EMA, mandate ECG monitoring during clinical trials to ensure the safety of drugs and protect against harmful cardiac events.
Holter monitors, in particular, contribute to the growth of this market by offering continuous monitoring (usually 24-48 hours), making them crucial for detecting arrhythmias that might not be visible in standard ECG exams. This extended monitoring capability is a significant advantage for both clinical trials and ongoing patient care, especially for those with known cardiovascular conditions like atrial fibrillation or ventricular arrhythmias. The growing demand for non-invasive, accurate, and continuous cardiac monitoring further fuels the growth of ECG and Holter monitor services.
End Use Insights
Based on the end use segment, Contract Research Organizations (CROs) was dominating, largely due to their integral role in supporting pharmaceutical and biopharmaceutical companies during the drug development process. While both pharmaceutical & biopharma companies and CROs contribute significantly to the market, CROs have become the primary drivers due to their specialized expertise in clinical trials, regulatory compliance, and patient monitoring. CROs act as intermediaries between drug developers and regulatory agencies, providing the necessary infrastructure, resources, and specialized services for comprehensive cardiac safety testing.One of the key factors contributing to the dominance of CROs is their ability to manage and execute large-scale clinical trials that are required to assess the safety and efficacy of new drugs, particularly in the context of cardiovascular conditions. Cardiac safety testing is a critical component of these trials, as any new drug, especially those targeting cardiovascular diseases, must undergo rigorous monitoring to ensure it does not cause adverse cardiac events such as arrhythmias, prolonged QT intervals, or other heart-related complications. CROs specialize in designing, managing, and executing clinical trials, and their deep expertise in this area enables pharmaceutical and biopharmaceutical companies to meet regulatory requirements while ensuring patient safety. They also provide the necessary infrastructure for conducting these trials, including patient recruitment, data collection, and real-time monitoring.
CROs are also highly skilled at navigating the regulatory complexities involved in drug development. Regulatory bodies such as the U.S. Food and Drug Administration (FDA), European Medicines Agency (EMA), and others set stringent guidelines for drug testing, particularly for drugs targeting cardiovascular diseases, where cardiac safety is of paramount concern. These agencies require comprehensive cardiac safety data before approving a new drug for market release. Given their in-depth knowledge of regulatory requirements and their experience in conducting clinical trials across different regions, CROs are in high demand by pharmaceutical and biopharmaceutical companies. The need for CROs to handle this complex regulatory framework, which involves managing multiple protocols and ensuring compliance across different jurisdictions, has fueled their dominance in the cardiac safety services market.
Regional Insights
North America was dominating the market, driven by a combination of factors including advanced healthcare infrastructure, strong regulatory frameworks, high investment in pharmaceutical and biopharmaceutical industries, and a rising prevalence of cardiovascular diseases. The U.S. and Canada, in particular, are leading players in this market, with their well-established healthcare systems, robust research capabilities, and significant demand for cardiac safety monitoring services.One of the key factors contributing to North America’s dominance in the cardiac safety services market is the presence of a large number of pharmaceutical and biopharmaceutical companies. The United States is home to some of the world's largest and most influential pharmaceutical companies, many of which are at the forefront of cardiovascular drug development. These companies conduct a substantial number of clinical trials, including those focused on new cardiovascular therapies, which require rigorous cardiac safety testing.
Regulatory agencies like the U.S. Food and Drug Administration (FDA) have stringent guidelines and safety standards for drugs, especially those targeting cardiovascular conditions. This has led to an increased demand for cardiac safety services in the region, as pharmaceutical companies must comply with these regulations to bring new drugs to market. The FDA’s emphasis on comprehensive cardiac safety assessments, particularly for drugs with potential arrhythmic effects, has fueled the growth of cardiac safety testing services, including ECG monitoring, Holter monitoring, and cardiovascular imaging.
Recent Developments
In May 2024, Vivalink unveiled an advanced technology solution for Holter monitoring and Mobile Cardiac Telemetry (MCT). This innovative solution merges remote patient monitoring (RPM) technologies with sophisticated arrhythmia detection algorithms.In March 2024, Wellysis, a digital healthcare company stemming from Samsung, partnered with Artella Solutions to launch a remote cardiac monitoring service in the U.S. The service features the FDA-approved S-Patch ExL device, which provides continuous monitoring for up to 14 days using a single coin battery. This collaboration expands Wellysis' portfolio, enhancing its offerings in Extended Holter and Mobile Cardiac Telemetry (MCT) solutions.
In March 2024, Medicalgorithmics has begun providing cardiac safety services for a groundbreaking clinical trial of a new medication. Currently in the drug dosing phase, the trial leverages Medicalgorithmics' advanced AI software and ECG diagnostic devices. This initiative is part of Project OATD-01, conducted for the Polish company Molecure and managed by Simbec-Orion. It marks a significant step forward for Medicalgorithmics as it expands its presence in the field of cardiac safety in clinical trials.
In October 2023, Dozee introduced its advanced ambulatory monitoring system, the 'Dozee Pro Ex,' which includes wireless wearable sensors to continuously track vital signs such as blood pressure, ECG rhythm, oxygen saturation, heart rate, temperature, and respiration rate. The system features an AI-powered early warning mechanism that monitors trends in vital parameters and provides timely alerts for potential clinical deterioration.
Key Market Players
Medpace, Inc.IQVIA Holdings Inc.
Thermo Fisher Scientific Inc.
Charles River Laboratories International, Inc.
WuXi AppTec Co., Ltd.
Nova Research Laboratories LLC
Laboratory Corporation of America Holdings
Koninklije Philips N.V.ICON Plc
Richmond Pharmacology Limited
Report Scope:
In this report, the Global Cardiac Safety Services Market has been segmented into the following categories, in addition to the industry trends which have also been detailed below:Cardiac Safety Services Market, By Service:
- ECG/Holter Monitors
- Blood Pressure Monitors
- Cardiovascular Imaging
- Others
Cardiac Safety Services Market, By Type:
- Integrated
- Standalone
Cardiac Safety Services Market, By End Use:
- Pharma & Biopharma Companies
- CROs
- Others
Cardiac Safety Services Market, By Region:
- North America
- United States
- Canada
- Mexico
- Europe
- France
- United Kingdom
- Italy
- Germany
- Spain
- Asia-Pacific
- China
- India
- Japan
- Australia
- South Korea
- South America
- Brazil
- Argentina
- Colombia
- Middle East & Africa
- South Africa
- Saudi Arabia
- UAE
Competitive Landscape
Company Profiles: Detailed analysis of the major companies present in the Global Cardiac Safety Services Market.Available Customizations:
With the given market data, the publisher offers customizations according to a company's specific needs. The following customization options are available for the report.Company Information
Detailed analysis and profiling of additional market players (up to five).This product will be delivered within 1-3 business days.
Table of Contents
Companies Mentioned
- Medpace, Inc.
- IQVIA Holdings Inc.
- Thermo Fisher Scientific Inc.
- Charles River Laboratories International, Inc.
- WuXi AppTec Co., Ltd.
- Nova Research Laboratories LLC
- Laboratory Corporation of America Holdings
- Koninklije Philips N.V.
- ICON Plc
- Richmond Pharmacology Limited
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 182 |
| Published | February 2025 |
| Forecast Period | 2024 - 2030 |
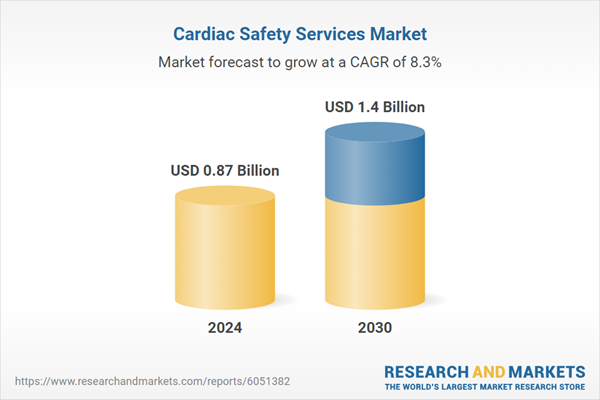
| Estimated Market Value ( USD | $ 0.87 Billion |
| Forecasted Market Value ( USD | $ 1.4 Billion |
| Compound Annual Growth Rate | 8.2% |
| Regions Covered | Global |
| No. of Companies Mentioned | 10 |