Global Ventricular Assist Devices Market - Key Trends & Drivers Summarized
How Are Technological Innovations Shaping the Ventricular Assist Devices Market?
Technological innovations are significantly shaping the ventricular assist devices (VADs) market, bringing about transformative changes in patient care and outcomes. Advances in medical technology have led to the development of smaller, more efficient, and durable VADs that offer improved performance and patient comfort. Modern VADs incorporate advanced sensors and automation, enabling real-time monitoring and adjustments, which enhance the precision and efficacy of the devices. Moreover, the integration of wireless technology allows for remote monitoring, providing healthcare providers with the ability to track patient health metrics and device performance from a distance. These innovations not only improve the quality of life for patients with severe heart conditions but also reduce the risk of complications and readmissions, thereby contributing to the growing adoption of VADs in clinical practice.What Role Do Regulatory Approvals and Clinical Trials Play in Market Dynamics?
Regulatory approvals and clinical trials play a crucial role in the dynamics of the ventricular assist devices market. Obtaining regulatory approval from agencies such as the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) is a critical step for VAD manufacturers. These approvals are based on rigorous clinical trials that demonstrate the safety and efficacy of the devices. Successful clinical trials and subsequent approvals can significantly boost market confidence and adoption rates. Additionally, ongoing clinical research and post-market surveillance studies provide valuable data on long-term outcomes and potential areas for improvement. Regulatory frameworks also ensure that VADs meet high standards of quality and performance, which is essential for gaining the trust of healthcare providers and patients. As new VADs continue to receive regulatory clearance and demonstrate positive clinical outcomes, the market is expected to expand further.How Are Market Dynamics and Competitive Strategies Influencing Growth?
The ventricular assist devices market is highly competitive, with several key players striving to enhance their market position through innovation and strategic initiatives. Companies are investing heavily in research and development to create next-generation VADs that offer superior functionality and reliability. Strategic partnerships, mergers, and acquisitions are also common, enabling companies to expand their technological capabilities and market reach. For instance, collaborations between VAD manufacturers and healthcare providers can facilitate the development of customized solutions tailored to specific patient needs. Additionally, companies are focusing on expanding their product portfolios to include devices for a broader range of heart conditions, such as right ventricular assist devices (RVADs) and total artificial hearts (TAHs). Marketing strategies emphasizing the clinical benefits and patient outcomes associated with advanced VADs are also crucial for driving market growth. The competitive landscape, characterized by constant innovation and strategic moves, is a significant factor propelling the market forward.What Are the Key Drivers of Growth in the Ventricular Assist Devices Market?
The growth in the ventricular assist devices market is driven by several factors. One of the primary drivers is the increasing prevalence of heart failure and other cardiovascular diseases, which creates a substantial demand for effective treatment options. Technological advancements, such as the development of more compact and efficient VADs, are enhancing device performance and patient comfort, thereby boosting adoption rates. Regulatory approvals and successful clinical trials that demonstrate the safety and efficacy of VADs further encourage their use in clinical settings. Additionally, the growing awareness and acceptance of VADs as a viable treatment option among healthcare providers and patients contribute to market expansion. The integration of remote monitoring technologies and the shift towards personalized medicine are also driving the demand for advanced VADs. Furthermore, strategic initiatives by market players, including research and development investments and partnerships, are fostering innovation and expanding the market.Report Scope
The report analyzes the Ventricular Assist Devices market, presented in terms of market value (US$ Thousand). The analysis covers the key segments and geographic regions outlined below.- Segments: Product (Left Ventricular Assist Devices (LVADs), Biventricular Assist Devices (BIVADs), Right Ventricular Assist Devices (RVADs), Other Products); Design (Implantable Ventricular Assist Device, Transcutaneous Ventricular Assist Device); Application (Destination Therapy, Bridge to Transplant, Other Applications).
- Geographic Regions/Countries:World; United States; Canada; Japan; China; Europe (France; Germany; Italy; United Kingdom; Spain; Russia; and Rest of Europe); Asia-Pacific (Australia; India; South Korea; and Rest of Asia-Pacific); Latin America (Argentina; Brazil; Mexico; and Rest of Latin America); Middle East (Iran; Israel; Saudi Arabia; United Arab Emirates; and Rest of Middle East); and Africa.
Key Insights:
- Market Growth: Understand the significant growth trajectory of the Left Ventricular Assist Devices (LVADs) segment, which is expected to reach US$10.6 Billion by 2030 with a CAGR of a 15.8%. The Biventricular Assist Devices (BIVADs) segment is also set to grow at 16% CAGR over the analysis period.
- Regional Analysis: Gain insights into the U.S. market, valued at $1.5 Billion in 2024, and China, forecasted to grow at an impressive 20.9% CAGR to reach $3.1 Billion by 2030. Discover growth trends in other key regions, including Japan, Canada, Germany, and the Asia-Pacific.
Why You Should Buy This Report:
- Detailed Market Analysis: Access a thorough analysis of the Global Ventricular Assist Devices Market, covering all major geographic regions and market segments.
- Competitive Insights: Get an overview of the competitive landscape, including the market presence of major players across different geographies.
- Future Trends and Drivers: Understand the key trends and drivers shaping the future of the Global Ventricular Assist Devices Market.
- Actionable Insights: Benefit from actionable insights that can help you identify new revenue opportunities and make strategic business decisions.
Key Questions Answered:
- How is the Global Ventricular Assist Devices Market expected to evolve by 2030?
- What are the main drivers and restraints affecting the market?
- Which market segments will grow the most over the forecast period?
- How will market shares for different regions and segments change by 2030?
- Who are the leading players in the market, and what are their prospects?
Report Features:
- Comprehensive Market Data: Independent analysis of annual sales and market forecasts in US$ Million from 2024 to 2030.
- In-Depth Regional Analysis: Detailed insights into key markets, including the U.S., China, Japan, Canada, Europe, Asia-Pacific, Latin America, Middle East, and Africa.
- Company Profiles: Coverage of players such as Abbott Laboratories, Inc., Abiomed, Inc., Berlin Heart GmbH, BiVACOR, Inc., Calon Cardio-Technology Ltd. and more.
- Complimentary Updates: Receive free report updates for one year to keep you informed of the latest market developments.
Some of the 28 companies featured in this Ventricular Assist Devices market report include:
- Abbott Laboratories, Inc.
- Abiomed, Inc.
- Berlin Heart GmbH
- BiVACOR, Inc.
- Calon Cardio-Technology Ltd.
- Cardiobridge GmbH
- Evaheart, Inc.
- HeartWare
- Jarvik Heart, Inc.
- Medtronic PLC
- ReliantHeart, Inc.
- SynCardia Systems LLC
- TandemLife
- Terumo Corporation
This edition integrates the latest global trade and economic shifts into comprehensive market analysis. Key updates include:
- Tariff and Trade Impact: Insights into global tariff negotiations across 180+ countries, with analysis of supply chain turbulence, sourcing disruptions, and geographic realignment. Special focus on 2025 as a pivotal year for trade tensions, including updated perspectives on the Trump-era tariffs.
- Adjusted Forecasts and Analytics: Revised global and regional market forecasts through 2030, incorporating tariff effects, economic uncertainty, and structural changes in globalization. Includes historical analysis from 2015 to 2023.
- Strategic Market Dynamics: Evaluation of revised market prospects, regional outlooks, and key economic indicators such as population and urbanization trends.
- Innovation & Technology Trends: Latest developments in product and process innovation, emerging technologies, and key industry drivers shaping the competitive landscape.
- Competitive Intelligence: Updated global market share estimates for 2025, competitive positioning of major players (Strong/Active/Niche/Trivial), and refined focus on leading global brands and core players.
- Expert Insight & Commentary: Strategic analysis from economists, trade experts, and domain specialists to contextualize market shifts and identify emerging opportunities.
Table of Contents
Companies Mentioned (Partial List)
A selection of companies mentioned in this report includes, but is not limited to:
- Abbott Laboratories, Inc.
- Abiomed, Inc.
- Berlin Heart GmbH
- BiVACOR, Inc.
- Calon Cardio-Technology Ltd.
- Cardiobridge GmbH
- Evaheart, Inc.
- HeartWare
- Jarvik Heart, Inc.
- Medtronic PLC
- ReliantHeart, Inc.
- SynCardia Systems LLC
- TandemLife
- Terumo Corporation
Table Information
| Report Attribute | Details |
|---|---|
| No. of Pages | 372 |
| Published | January 2026 |
| Forecast Period | 2024 - 2030 |
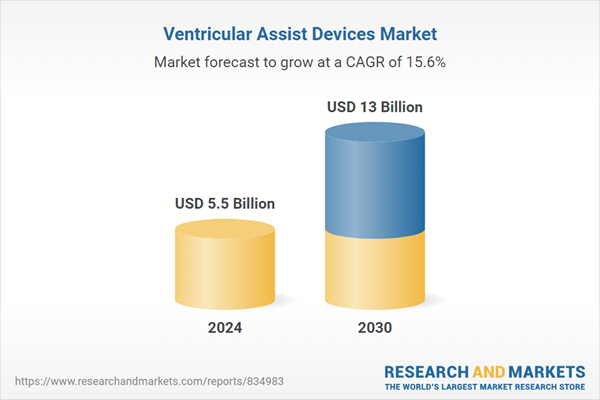
| Estimated Market Value ( USD | $ 5.5 Billion |
| Forecasted Market Value ( USD | $ 13 Billion |
| Compound Annual Growth Rate | 15.6% |
| Regions Covered | Global |